Unraveling Huntington's Disease
- Published25 Aug 2016
- Reviewed25 Aug 2016
- Author Jennifer Brummet
- Source BrainFacts/SfN
Nonhuman primate research is opening up new avenues of attack against the devastating neurodegenerative disorder Huntington’s disease.
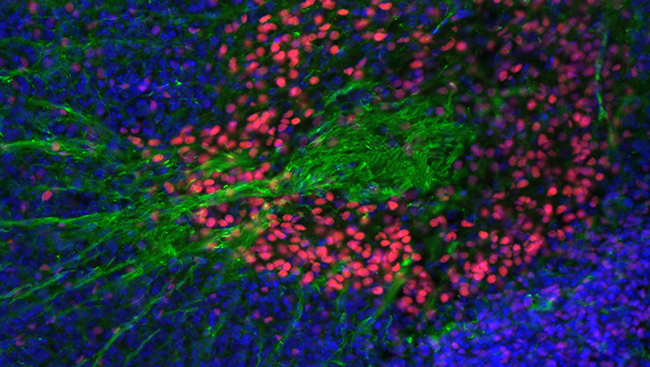
Huntington’s disease is associated with cell death in the basal ganglia, part of which is pictured here.
Huntington’s disease (HD) is a fatal, inherited disorder that destroys brain cells in a region of the brain involved in motor control. Caused by a mutation in the huntingtin gene, the disease impairs brain cells’ ability to function and causes them to degenerate as toxic proteins accumulate. Symptoms typically appear in the 40s and include uncontrolled movements called chorea, personality changes, depression, and problems with memory and judgment. Currently, there is no cure for the progressive disease that affects 30,000 Americans, and only one drug is FDA-approved to treat Huntington’s-associated chorea.
A major roadblock in the treatment and early intervention of HD has been the lack of an appropriate animal model. While scientists have developed rodent models with the mutant huntingtin gene, none of those truly mimic the motor, cognitive, and behavioral symptoms of the disease as they arise in humans. As a result, none serve as particularly useful models for developing desperately needed new therapeutics.
Nonhuman primate models offer greater promise for understanding and treating neurodegenerative diseases like HD because they are more similar to humans. The brains of rhesus macaque monkeys, for example, share anatomy and developmental trajectory with human brains. And unlike rodents, rhesus monkeys possess fine motor control and complete complex cognitive tasks, abilities that are progressively impaired in HD. Because HD is a progressive disease, animal models need to be studied over a long period of time. Rhesus monkeys are well suited to this because, although their brains develop similarly to humans’, they do so in a shortened timeframe — they live up to 20 years and reach puberty by age four.
In 2015, a team of researchers at the Yerkes National Primate Research Center at Emory University developed a rhesus monkey model for Huntington’s disease. The HD monkeys displayed motor and cognitive impairments at 16 months of age. By 24 months of age, they showed signs of neurodegeneration in brain scans and developed the involuntary muscle contractions characteristic of Huntington’s disease. Such pathological changes are not observed in rat and mouse models of HD.
In short, these HD monkeys showed progressive clinical symptoms very similar to what is seen in people with HD, making them a critical nonhuman primate model for treating and preventing this genetic disease. “The promise a nonhuman primate model holds for developing treatments could alter the course of Huntington’s disease,” says Anthony Chan who led the research team. “Right now, we can address the symptoms of the disease, but we want to do more. A nonhuman primate model should be able to help us better help others.”
Already, a drug designed to silence the mutant gene that synthesizes the toxic Huntington’s disease protein has been tested in monkeys and is now in clinical trials in humans. This is just one example of how research in nonhuman primates facilitates finding potential treatments and cures for debilitating diseases.
CONTENT PROVIDED BY
BrainFacts/SfN