The Mind-Bending Power of Bacteria
- Published8 Jan 2015
- Reviewed8 Jan 2015
- Source The Kavli Foundation
Our bodies are home to a vast ecosystem of microbes — the microbiome — that has a powerful effect on the brain. Three brain researchers discuss the emerging connection between the brain and the gut, and whether microbes may help treat brain disorders.
On January 15, 2015, neuroscientist Christopher Lowry discussed the emerging science that's connecting the microbiome – the community of microbes that inhabit the body – with brain health, including whether we can treat common brain disorders through the gut.
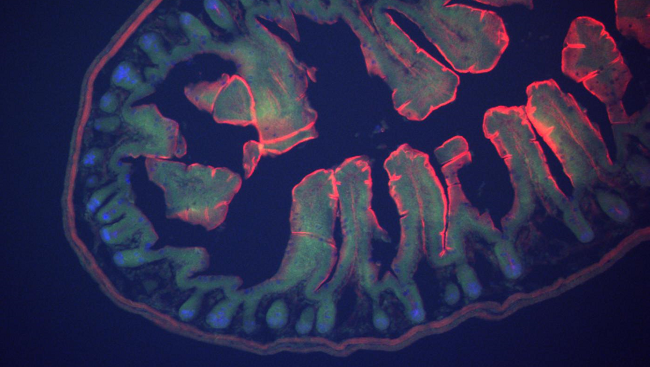
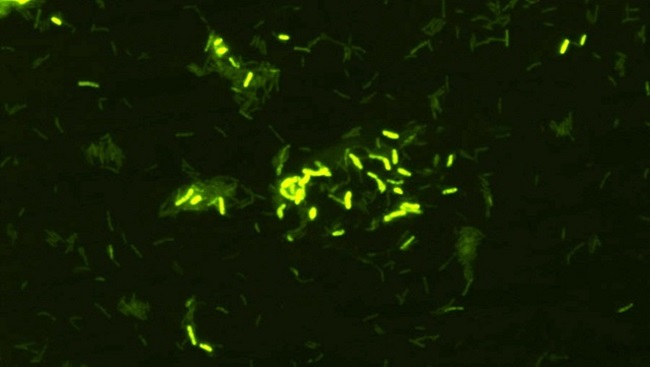
OUR RELATIONSHIP WITH MICROBES is usually described as an arms race, with humans and bacteria on opposing sides. But, as new research is showing, a better metaphor is “brothers-in-arms.” The 10,000 or so different types of microbes, including bacteria, viruses and fungi, that inhabit our bodies form a balanced ecosystem called the microbiome. They are mostly beneficial – as integral to our survival as we are to theirs – helping us to digest food, make vitamins, combat infection and much more.
In fact, evidence accumulated in the last five to 10 years shows that these microbes, which predominantly live in the gastrointestinal tract, shape the development and function of the brain. They influence a range of complex human behaviors, including learning and memory, mood and emotion, and appetite and satiety. They have also been linked to disorders of the central nervous system including anxiety, depression, autism and multiple sclerosis, which may be a consequence of an ecosystem that has fallen out of balance.
Three researchers at the forefront of research on the microbiome-brain connection recently spoke with The Kavli Foundation about how microbes communicate with the brain and whether we can modify the gut microbiome to treat disorders of the brain and mind.
The participants were:
TRACY BALE, PhD - is a professor of neuroscience at the School of Veterinary Medicine andPerelman School of Medicine at the University of Pennsylvania. She is studying the effects of early prenatal stress on fetal brain development and has shown that they are partly mediated by the microbiome.
CHRISTOPHER LOWRY, PhD - is an associate professor in the Department of Integrative Physiology at the Center for Neuroscience at the University of Colorado Boulder and director of the Behavioral Neuroendocrinology Laboratory. Lowry is developing new strategies to prevent and treat anxiety and depression, including the use of beneficial microbes that live in the gut.
SARKIS MAZMANIAN, PhD - is the Louis & Nelly Soux Professor of Microbiology at the California Institute of Technology and a 2012 MacArthur Fellow. A microbiologist and immunologist by training, Mazmanian studies how the brain, the immune system and the microbiome interact in health and disease, including autism spectrum disorder.
The following is an edited transcript of a roundtable discussion, which took place via teleconference on December 1, 2014. The participants have been provided the opportunity to amend or edit their remarks.
THE KAVLI FOUNDATION: The idea that the microbes living in our gut have an effect on the brain is a relatively new one. What set you down the path to studying this relationship? And how has your thinking evolved since then?
SARKIS MAZMANIAN: For more than a decade, my laboratory has been studying the interaction between microbes and the immune system. I took this path in the last five years because, through conversations with neuroscientists here at Caltech, I realized there are many parallels between the immune and nervous systems. For example, immune cells and neurons produce and sense many of the same chemicals. Since microbes were having such a profound effect on the immune system, I wondered whether they were having an effect on the brain, too.
I thought that we would find that microbes interact with the brain via the immune system. But the data we’ve generated so far have shown that microbes interact with the brain by producing molecules that impact behavior without altering the immune system. Though we haven’t ruled out an immune link, we have discovered mechanisms by which microbial molecules may directly interact with the nervous system.
CHRISTOPHER LOWRY: My lab has been studying interactions between bacteria, the nervous system and emotional behavior for about 15 years. We’ve found, for example, that mice exposed to an inactivated soil bacterium called Mycobacterium vaccae increase production of the neurotransmitter serotonin in the brain, which has antidepressant-like effects.
The turning point for looking at gut-microbiome-brain interactions in my lab really came with our first collaboration with Rob Knight, who leads the American Gut Project here at CU-Boulder. Together, we’ve been investigating ways of modulating the immune system to prevent stress-related psychiatric disorders, such as anxiety and mood disorders. Although this work is still in progress, it’s clear that the microbiota plays an important role in stress-induced chronic anxiety, at least in animal models.
TRACY BALE: My lab works on the impact of stress during pregnancy on the developing brain using a mouse model of early maternal stress. There was a Radiolab episode on NPR that provoked a conversation in the lab about the vaginal microbiome, which is the main source of the bacteria that first populate a newborn baby’s gut. We started thinking about the environmental factors, such as stress, that could change the vaginal microbiome and wondering if that would have an impact on our model of brain development. It blossomed from there because the relationship between a newborn’s gut microbiome and his or her mother’s vaginal microbiome is almost one-to-one, with a newborn’s microbiome changing in direct response to its mother’s. We wondered, how does this interaction alter the way a baby’s brain develops in our mouse model of early prenatal stress? It turns out that stress changes the levels of Lactobacillus, a gut-dwelling lactic acid-producing bacteria that affects the brain’s chemistry, in both mothers and their offspring.
TKF: What are the big questions about the gut-microbiome-brain connection that researchersare trying to answer right now?
BALE: Now that we know the microbiome changes in response to stress, I think the field is trying to understand the processes by which the gut microbiome alters the brain.
LOWRY: I totally agree. The big question right now is how the microbiome exerts its effects on the brain. Some questions we’re all still trying to answer include the composition of the gut microbiota, its effects on the permeability of the lining of the gastrointestinal tract, its effects on whole-body inflammation, and its effects on neuronal signaling from the gut to the brain.
MAZMANIAN: Another big question is whether we can treat brain disorders, such as autism, by aiming therapies at the gut. One of the barriers to treating psychiatric and neurological disorders is that we often don’t understand the underlying disease mechanisms; in other words not only what to target but where those targets are in the body. If the therapeutic targets are in the brain then it becomes particularly challenging because of the blood-brain barrier, a network of blood vessels that protects the brain from harmful substances. But if a neurological condition actually originates in the gut, which we believe is the case for some individuals with autism, then delivering therapeutics is much easier. In my lab, we’ve been able to alter some of the symptoms associated with autism, such as repetitive behaviors, in mice by feeding them specific bacterial. These bacteria modulate molecules in the gut and in the blood that affect the nervous system.
TKF: That leads me to my next question. Sarkis, how do you think the microbes in the gut are communicating with the brain and driving behavior? Have your experiments using mice with autism-like symptoms offered any clues?
MAZMANIAN: There are at least three ways gut microbes are communicating with the brain: the first is directly through the vagal nerve, which connects the network of nerves in the gut to the brain; the second is through circulating immune cells that are primed, or educated, in the gut and then travel to the brain; and the third may be metabolites, molecules that are produced by microbes in the gut that enter the blood and circulate to regions of the brain where they affect behavior. We’ve shown, for example, that a metabolite produced by gut bacteria is sufficient to cause behavioral abnormalities associated with autism and with anxiety when it is injected into otherwise healthy mice. This suggests that microbial molecules may connect the gut to the brain via the circulatory system.
BALE: Sarkis covered most of what people currently understand about what’s happening in the gut. But keep in mind that there’s a developmental window that is also important. We’ve shown in our mouse model of early maternal stress, for example, that short-term changes to the microbiome during a critical period of development can result in brain changes.
TKF: Has anyone actually traced changes in the human brain back to the microbiome?
MAZMANIAN: Emeran Mayer and his colleagues at the University of California, Los Angeles, used functional magnetic resonance imaging, or fMRI, to look at the effects of specific microorganisms on brain activity. He showed that treating healthy people with fermented milk products containing probiotics, or healthy bacteria, altered brain activity in regions linked to emotion.
BALE: Developmentally, that’s a harder question, because you can’t do controlled experiments in newborns. But there are plenty of studies ongoing in Europe and in Canada where researchers are giving vaginal lavages to the newborns delivered by C-section to ensure that they are getting a nice dose of their mother’s microbiome.
TKF: Tracy, you’ve talked about the close connection between a mother’s vaginal microbiome, which a baby encounters as it passes through the birth canal, and her newborn baby’s. Can you expand on how this natural form of inoculation helps lay the foundation for brain development?
BALE: There are key developmental windows when the brain is more vulnerable because it’s setting itself up to respond to the world around it. At the same time, as Sarkis pointed out, the gut microbiome is helping to prepare the baby’s immune system. So, if mom’s microbial ecosystem changes – due to infection, stress or diet, for example – her newborn’s gut microbiome will change too, and that can have a lifetime effect. It could alter how the gut, and the immune system within the gut, develops and how the brain develops.
What’s interesting, from our experiments on the effects of exposure to early maternal stress in mice, is that if you look at those babies in adulthood, their microbiome may be completely normalized, but if you then stress them or give them an immune challenge, you see big differences again in the way their bodies respond. So even acute exposure to prenatal stress seems to alter how an organism responds to changes in its environment in the long term.
TKF: I find that a little scary. Pregnant women already have so much to worry about.
MAZMANIAN: I can understand that reaction. Just the very concept that microbes can have profound effects on the brain may be hard for a lot of people to believe. But I tend to think that by understanding these effects, one can do something about the epidemics of anxiety, autism and attention deficit and hyperactivity disorder, also known as ADHD, that we’re seeing in today’s society. We’re learning that society may be doing things that are not in the best interest of a child’s health and by understanding those things we can change practices and prevent, for a lack of a better term, mistakes during critical periods of development. So my take is a little bit more positive.
BALE: I agree. I think that it’s an awareness more than a fear factor.
TKF: Christopher, you’ve suggested that a lack of exposure to the many bacteria we evolved with may be contributing to our ills, including psychiatric disorders such as depression and anxiety. If that’s the case, can we make up for the past?
LOWRY: There are certain psychiatric disorders like post-traumatic stress disorder, or PTSD, and depression that seem to be associated with increased inflammation, a ramped-up immune response in the body and in the brain. So microbes that can modulatethe immune system and limit inflammation may have some benefit, either by alleviating the symptoms of certain types of psychiatric disorders or, in the best possible scenario, preventing them. So yes, I think taking or being exposed to these types of bacteria during adulthood can be beneficial, although clearly their major influences are during development, as Tracy and Sarkis have pointed out.
TKF: Are you testing any probiotics for stress or depression?
LOWRY: Our current studies use heat-killed bacteria rather than probiotics, which the World Health Organization defines as live microorganisms that confer a health benefit to the host when administered in adequate amounts. We have a series of studies that we’re getting ready to publish that suggest that certain types of heat-killed microbes can prevent syndromes that you would expect following chronic stress. We’re hoping to conduct similar studies using probiotics in animals and humans.
TKF: Have you changed your behavior as a result of your research into the connection between the microbiome and a healthy body and brain?
LOWRY: I’ve certainly altered mine. I eat more fresh vegetables, and we have a small garden at our house. It’s becoming clear from the American Gut Project that an important source of gut microbial diversity is the number of different plants we eat. Fresh vegetables are a significant source of bacteria For example, the leaves of a spinach plant are estimated to harbor more than 800 different species of microbes. These are microbes that you can’t sterilize from the plant because they’re actually inside of it. And so having access to this kind of microbial diversity through the things that we eat is probably very important. I’ve also added sand from ocean beaches to my children’s sandbox, I let my kids swim in lakes, and we take probiotics.
MAZMANIAN: As we all know, it’s still the very early days in terms of trying to understand the effects of the microbiome, even for inflammatory bowel disease or obesity, which have been studied much more than any neurological condition. That being said, as Chris mentioned, diet is a major driver of microbiome configuration and so I think that there is enough reason to believe that specific foods “are better” for the microbiome than others: eating a high fiber diet with lots of fruits and vegetables, resistant starches such as those from seeds and nuts, and, in general, a diet that is closer to the one we evolved to eat, including foods that were available before agriculture and fast food restaurants came along. So what I’ve done is nothing really dramatic.
BALE: As a parent, I would also say I’ve spent a lot more time thinking before allowing my child to take antibiotics. There’s no disputing their utility or efficacy but we shouldn’t just throw antibiotics at every illness.
TKF: Sarkis, you’ve called recent discoveries “harbingers of extensive, currently undescribed links between gut bacteria and the nervous system.” Can you speculate on what other links between the gut microbiome and the brain might emerge? Are we going to find that the microbiome also plays a role in cognition or neurodegenerative diseases, for example?
MAZMANIAN: It’s already been shown that cognition in germ-free mice is reduced in comparison to normal mice. While the microbiome has not yet been implicated in neurodegeneration, there’s a lot of speculation about this issue because there are data showing that a Mediterranean diet protects against Alzheimer’s, a neurodegenerative disease. And because we know diet helps shape the microbiome, one can imagine that eating a Mediterranean diet could affect the microbiome in a way that protects against neurodegeneration. We have a program in the lab that’s very, very early looking at Alzheimer’s and Parkinson’s diseases in mouse models, but there’s nothing solid to report yet.
There are other anecdotal reasons to believe that neurodegeneration may have some connection to the microbiome, or vice versa, because the three major neurodegenerative disorders – Alzheimer’s, Parkinson’s and amyotrophic lateral sclerosis, or ALS – all have a GI component to them. In fact, many patients who are ultimately diagnosed with Parkinson’s are known to have had GI disturbances decades before they develop motor symptoms. And a recent study showed that patients with Parkinson’s disease have different microbiomes than matched controls. While this is an exciting finding, this kind of study needs to be interpreted with caution as it is an association and does not prove a causative link. So there are flash bulbs going off in the dark, suggesting that very complex neurodegenerative disorders may be linked to the microbiome. But once again this is very speculative. These seminal findings, the flash bulbs, are only just beginning to illuminate our vision of the gut-microbiome-brain connection.
TKF: In addition to more funding, what would help accelerate progress in the field?
LOWRY: There are regulatory issues related to giving live or dead bacteria for treatment of psychiatric and neurological disorders, and those are significant issues that will have to be dealt with.
BALE: Another is that not all neuroscientists have embraced this idea. It’s definitely getting better: I just spoke at the American College of Neuropsychopharmacology meeting; it’s quite surprising that they had a session on microbiome this year. But I can tell you that there are still vast numbers of people, especially in psychiatry, who don’t believe this is real. And I think that is a hurdle.
MAZMANIAN: I have a similar story. We had a session on the microbiome at the annual meeting of the Society for Neuroscience this year but it was our third year submitting it. I think that’s a sign there is a sea change taking place in the field. With that being said, if you talk to most neuroscientists, they have a hard time finding any credibility in the link between the microbiome and neurobiology. It’s still a very, very new concept with much more experimental validation necessary, and honestly I have no issue with that.
BALE: I agree. I think the field is now at the point where we’ve established the validity of our models and are beginning to show how the microbiome affects the brain. That is when the big change in attitude will happen. But it’s going to require some hard-core studies of the mechanisms involved.
MAZMANIAN:I think the skepticism is healthy. It keeps us honest and makes sure that the science remains rigorous. At the same time, I’ve been surprised and gratified by the fact that our work hasn’t received widespread rejection by the neuroscience community. It certainly hasn’t received widespread acceptance either, but when I discuss my research with card-carrying neuroscientists they seem to be quite open-minded about the possibility. It’s not that they believe it, but they’re intrigued to see more.
BALE: Right. The fecal transplant jokes have subsided!
— Lindsay Borthwick (Winter 2014)
CONTENT PROVIDED BY

The Kavli Foundation
Also In Diet & Lifestyle
Trending
Popular articles on BrainFacts.org