Sending Information: Synapses and Neurotransmission
- Reviewed30 Nov 2022
- Author Diane A. Kelly
- Source BrainFacts/SfN
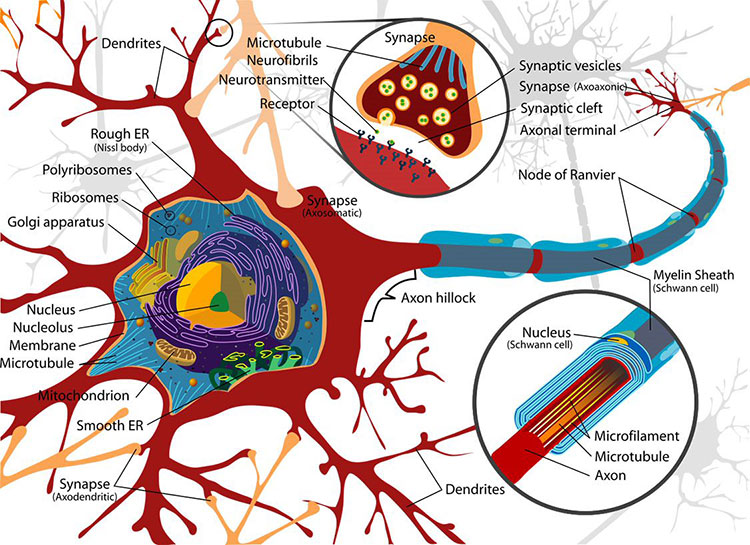
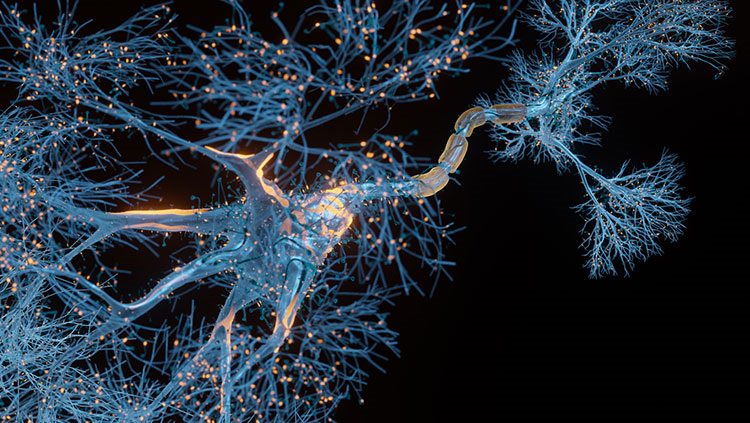
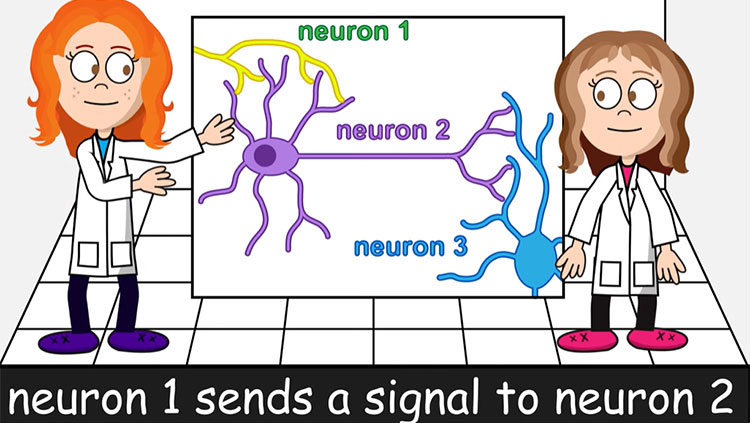
Neurons pass information to each other in a process called neurotransmission. Signals are passed from one neuron to the next at junctions called synapses. In most circuits, a synapse includes the end of an axon, the dendrite of an adjacent neuron, and a space between the two called the synaptic cleft. Amazingly, this separation between neurons was only verified (by electron microscopy) in the 1950s. The cleft is wide enough that electrical signals can’t directly impact the next neuron; rather, chemical signals called neurotransmitters cross the synapse. This process is called neurotransmission.
When an action potential arrives at the axon terminal, the voltage change triggers ion channels in the membrane to open, which lets calcium ions flow into the cell. When the calcium ions bind to packages of neurotransmitter molecules called synaptic vesicles, the vesicles fuse with the cell membrane at the axon terminal and empty their contents into the synaptic cleft. Afterwards, pieces of axon terminal membrane cycle back into the soma as new vesicles, which are refilled with neurotransmitter molecules.
Many substances act as neurotransmitters, including amino acids, gases, small organic chemicals, and short peptides. Neurons can synthesize small non-peptides like dopamine or acetylcholine inside the axon terminal. But an axon terminal doesn’t contain the molecular machinery for building proteins, so peptide-based neurotransmitters are built in the ribosome-rich space of the cell body. Vesicles containing neurotransmitter “cargos” bud off from the wall of the Golgi apparatus — the cell’s protein-packaging organelle — then bind to proteins called kinesins that work their way down the axon along microtubules, filamentous parts of the cellular skeleton.
After neurotransmitters are released from an axon terminal, they drift across the synaptic cleft until they reach the outer surface of the dendrite, a region that looks thick or dense in highly magnified images. This region, the postsynaptic density, has a high concentration of neurotransmitter receptors. Many different molecules act as neurotransmitters, and each one fits into specific receptors like a key fits a lock. Receptors are linked to ion channels in such a way that, when neurotransmitter molecules dock on their receptors, they open those channels, altering the voltage across the postsynaptic membrane. Local glial cells (astrocytes) mop up any excess neurotransmitters at the synapse. This process prevents them from continuously activating receptors.
There are two broad types of receptors on the postsynaptic membrane. In an ionotropic receptor, a neurotransmitter binds directly to part of an ion channel. The channel is normally closed; the receptor protein changes its shape when the neurotransmitter attaches, widening the tunnel in the center of the ion channel so that ions can move through. Metabotropic receptors are more complex. The receptor and the ion channel are different proteins located at a distance from one another, but they are linked by a cascade of biochemical steps that are triggered when a neurotransmitter binds to the receptor. This response is less rapid and activates a series of events inside the postsynaptic cell. The result may be opening an ion channel some distance away or activating other intracellular molecules.
Neurotransmitter molecules only bind to their receptors for a short time. Once they detach, the ion channels return to their resting state and stop altering the charge across their membrane. The neurotransmitters are either broken down or reabsorbed by the axon terminal in a process called reuptake.
Excitatory and inhibitory neurons can be identified by the specific neurotransmitters that they make. Excitatory neurons make neurotransmitters that open ion channels that depolarize the dendrite’s membrane; inhibitory neurons make neurotransmitters that hyperpolarize it. The brain’s most common excitatory neurotransmitter is glutamate; the brain’s most common inhibitory neurotransmitter is gamma-aminobutyric acid (GABA).
Glutamate is an amino acid used as a neurotransmitter by approximately half the excitatory synapses in the brain. It can bind to several types of ionotropic receptors; the most important of these are AMPA receptors and NMDA receptors. When activated, the action of AMPA receptors is fast and brief; NMDA receptors activate more slowly, particularly in response to waves of multiple action potentials. Interactions between these receptors appear to be important in learning and memory.
GABA is the brain’s most important inhibitory neurotransmitter. It binds to two groups of receptors; one group is ionotropic, the other metabotropic. Ionotropic GABA receptors have ion channels that let negatively charged chloride ions enter the cell. Metabotropic GABA receptors open ion channels that release potassium ions. In both instances, ion movement pushes membrane potential downward and inhibits a neuron from firing.
Adapted from the 8th edition of Brain Facts by Diane A. Kelly.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Albuixech-Crespo, B., López-Blanch, L., Burguera, D., Maeso, I., Sánchez-Arrones, L., et al. (2017). Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLOS Biology, 15(4): e2001573. https://doi.org/10.1371/journal.pbio.2001573
Barton, R. A., & Venditti, C. (2014). Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Current Biology, 24(20), 2440–2444. https://doi.org/10.1016/j.cub.2014.08.056
Bekkers, J. M. (2011). Pyramidal neurons. Current Biology, 21(24), PR975. https://doi.org/10.1016/j.cub.2011.10.037
Belkhiria, C., Driss, T., Habas, C., Jaafar, H., Guillevin, R., & de Marco, G. (2017). Exploration and Identification of Cortico-Cerebellar-Brainstem Closed Loop During a Motivational-Motor Task: an fMRI Study. The Cerebellum, 16, 326–339. https://doi.org/10.1007/s12311-016-0801-1
Bromfield, E. B., Cavazos, J. E., Sirven, J. I. (2006). An Introduction to Epilepsy, https://www.ncbi.nlm.nih.gov/books/NBK2508/
Carpenter, R., & Reddi, B. (2012). Neurophysiology: A Conceptual Approach, 5th edition. Hodder Arnold: London.
Castro, A., Becerra, M., Manso, M. J., & Anadón, R. (2015). Neuronal organization of the brain in the adult amphioxus (Branchiostoma lanceolatum): A study with acetylated tubulin immunohistochemistry. The Journal of Comparative Neurology, 523(15), 2211–2232. https://doi.org/10.1002/cne.23785
Clarke, L. E., & Barres, B. A. (2013). Emerging roles of astrocytes in neural circuit development. Nature Reviews Neuroscience, 14, 311–321. https://doi.org/10.1038/nrn3484
Fain, G. L., & O’Dell T. J. (2014). Molecular and Cellular Physiology of Neurons, 2nd edition. Harvard University Press: Cambridge.
Forger, N. G. (2016). Epigenetic mechanisms in sexual differentiation of the brain and behaviour. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1688), 20150114. https://doi.org/10.1098/rstb.2015.0114
Frohlich, F. (2016). Network Neuroscience, 1st edition. Academic Press: London.
Guo, J. U., Ma, D. K., Mo, H., Ball, M. P., Jang, M. H., Bonaguidi, M. A., Balazer, J. A., Eaves, H. L., Xie, B., Ford, E., Zhang, K., Ming, G. L., Gao, Y., & Song, H. (2011). Neuronal activity modifies the DNA methylation landscape in the adult brain. Nature Neuroscience, 14, 1345–1351. https://doi.org/10.1038/nn.2900
Hammond, C. (2014). Cellular and Molecular Neurophysiology, 4th edition. Academic Press.
Human Brain. (2017). Allen Brain Atlas. Allen Institute for Brain Science. https://human.brain-map.org/
Lee, A., Fakler, B., Kaczmarek, L. K., & Isom, L. L. (2014). More Than a Pore: Ion Channel Signaling Complexes. The Journal of Neuroscience, 34(46), 15159–15169. https://doi.org/10.1523/JNEUROSCI.3275-14.2014
Noback, C. R. et al (eds.). (2005). The Human Nervous System: Structure and Function, 6th edition. Humana Press: Totowa NJ.
O'Muircheartaigh, J., Keller, S. S., Barker, G. J., & Richardson, M. P. (2015). White Matter Connectivity of the Thalamus Delineates the Functional Architecture of Competing Thalamocortical Systems. Cerebral Cortex, 25(11), 4477–4489. https://doi.org/10.1093/cercor/bhv063
Peer, M., Nitzan, M., Bick, A. S., Levin, N., & Arzy, S. (2017). Evidence for Functional Networks within the Human Brain's White Matter. The Journal of Neuroscience, 37(27), 6394–6407. https://doi.org/10.1523/JNEUROSCI.3872-16.2017
Pyka, M., & Cheng, S. (2014). Pattern Association and Consolidation Emerges from Connectivity Properties between Cortex and Hippocampus. PLOS ONE, 9(1), e85016. https://doi.org/10.1371/journal.pone.0085016
Saladin, K. (2015). Anatomy & Physiology: The Unity of Form and Function, 7th edition. McGraw Hill: New York.
Schneider, G. E. (2014). Brain Structure and its Origins: in Development and in Evolution of Behavior and the Mind. MIT Press: Cambridge.
Sheng, M., Kim, E. (2011). The postsynaptic organization of synapses. Cold Spring Harbor Perspectives in Biology, 3(12), a005678. https://pubmed.ncbi.nlm.nih.gov/22046028
Sporns, O. (2013). Structure and function of complex brain networks. Dialogues in Clinical Neuroscience, 15(3), 247–262. https://doi.org/10.31887/DCNS.2013.15.3/osporns
Verberne, A. J., Sabetghadam, A., & Korim, W. S. (2014). Neural pathways that control the glucose counterregulatory response. Frontiers in Neuroscience, 8(38). https://doi.org/10.3389/fnins.2014.00038
Wells, R. B. (2005). Cortical Neurons and Circuits: A Tutorial Introduction. https://webpages.uidaho.edu/rwells/techdocs/Cortical%20Neurons%20and%20Circuits.pdf
What to Read Next
Also In Cells & Circuits
Trending
Popular articles on BrainFacts.org