How Studying ‘Drug-seeking’ Habit and Compulsion in Rats Offers Addiction Treatment Insight
- Published13 Oct 2022
- Author Calli McMurray
- Source BrainFacts/SfN
Barry Everitt, the Society for Neuroscience’s president from 2019-2021, studies how drug seeking and use in vulnerable individuals spiral from recreational to compulsive in the development of addiction. When he began his work, most addiction research was focused on the pharmacological mechanisms of action of different addictive drugs in the brain, revealing that dopamine in the nucleus accumbens is a common mediator of their reinforcing effects.

However, Everitt, emeritus professor of behavioral neuroscience at the University of Cambridge, thought that “addiction is a disorder that embodies much more than drug reinforcement or reward, and the development of compulsive seeking and taking of drugs is at its core.” Using a combination of animal learning theory, systems, and cellular neuroscience approaches, he and his colleagues developed novel behavioral procedures to investigate the neural and psychological mechanisms involved.
It has long been known that otherwise innocuous environmental stimuli become associated with the effects of repeatedly taking addictive drugs like cocaine through Pavlovian conditioning; these ‘drug cues’ induce drug craving and relapse long after drug use has stopped. To study this, Everitt and collaborators developed a procedure in which rats would press a lever for long periods for an infusion of cocaine, but only if these cocaine-associated cues were presented contingent on their drug-seeking responses by acting as conditioned reinforcers.
Early on, this cocaine-seeking behavior is goal-directed and depends upon an amygdala-ventral striatal (nucleus accumbens) system in the brain. But after a few weeks of seeking drugs in this way, control over the behavior shifts from the ventral to the dorsal striatum. Everitt hypothesized and later showed that this reflected the development of a cocaine-seeking ‘habit.’ The behavior was no longer goal-directed, but instead, the rats’ seeking lever presses were elicited and maintained automatically by drug cues in the environment.
Everitt says, “the suggestion that drug seeking becomes habitual was, and probably is, not widely accepted, despite the compelling neural and psychological evidence in animals and humans; it seems counterintuitive.” How could someone seek drugs if not doing so because they want to? Well, “habits are fundamental to our daily lives,” Everitt says. “We all have them, though [we] may not recognize them as such.”
His favorite personal example concerns his routine. Monday through Friday, he leaves his home in Cambridge, United Kingdom, and walks to his lab. He unlocks the door, turns on his computer, checks his email, and gets to work. On Saturdays and Sundays, he leaves home and walks along the same route to pick up a newspaper at a shop just around the corner. But sometimes without a second thought, he finds himself on the weekend in his office at his lab computer instead of at the newspaper shop. To an observer, he may have looked goal-directed, but he wasn’t. Everitt’s brain kicked into autopilot — his body was just along for the ride. Everitt thinks the same type of automatic behavior is elicited in people who have repeatedly used drugs.
“Barry Everitt’s conceptualization is fascinating. If you boil it down, what he’s saying is that objects in the environment can become associated with the drug, and these objects can gain control over your behavior,” says Paul Kenny, professor of neuroscience and director of the Drug Discovery Institute at the Icahn School of Medicine at Mount Sinai. “Essentially, you become almost robotic because these programs of behavior are so intrinsically, inextricably intertwined into who you are as a drug user, that the behavior moves beyond your conscious control and is evoked simply by being exposed to stimuli in the environment.”
"...developing a drug-seeking habit does not itself equate with being addicted; at some point, the habit becomes extremely difficult to break and emerges as compulsive.”
In humans, drug-seeking behaviors entail actions more complex than pressing a lever. “That behavior might look purposeful, but it has become automatic. And there’s lots of things that we do like that: They look goal-directed, but it’s actually because they’ve been done so often, they’re not,” Everitt says — like checking your cell phone. “Initially, it’s to look to see if I’ve got a message, but in fact, it becomes automatic. Even when there’s nothing there, it repeats and repeats.”
Everitt notes: “But it’s important to understand that developing a drug-seeking habit does not itself equate with being addicted; at some point, the habit becomes extremely difficult to break and emerges as compulsive.” For this to happen, another factor must be involved. Everitt and collaborators proposed that an impairment in so-called ‘top-down’ cognitive control over habits as a consequence of chronic drug use is an important mechanism.
To study compulsive drug seeking, Everitt and his team developed a new and now increasingly adopted method in which rats would learn to press a lever to “take” cocaine but then learn to press another lever — the “seeking lever” — in order to gain access to the “taking lever” and a cocaine infusion. An unpredictable mild footshock punishment is then introduced as the outcome of seeking responses instead of the taking lever, so rats must run the risk of punishment to take cocaine. The important result of these experiments was that after a short history of cocaine seeking, all rats stopped seeking and abstained in the face of punishment. However, after a long history of cocaine, 20% or so of rats continued to seeking cocaine despite punishment; they became compulsive.
The Fingerprint of Addiction
This now much-replicated finding of a sub-population of individuals that are vulnerable to developing compulsive cocaine seeking has its clear parallel among humans who initially experiment with drugs — since only about 20% go on to become addicted. Understanding the neural and psychological basis of this vulnerability is a major research topic at Cambridge and other labs worldwide.
As habits emerge, there is a shift in the neural circuitries involved, including that from the ventral to the dorsal striatum. Functional activity of areas of the prefrontal cortex that underlie top-down control over behavior are also decreased after chronic cocaine use. Compulsive drug seeking may result, therefore, from impaired control over the goal-directed system, or enhanced activity of the habit system, or a combination of both as a result of prefrontal hypoactivity. There is emerging evidence for animal and human studies.
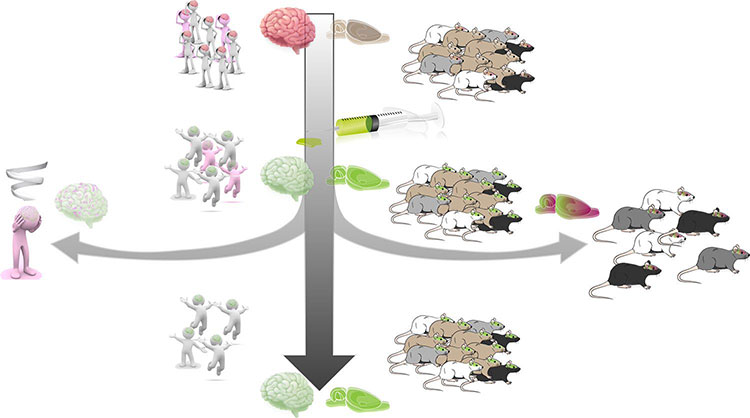
“It’s important now to know whether such propensities for brain-behavior changes exist before, rather than only being a consequence of drug use,” Everitt says. In other words, are there pre-existing brain signatures of vulnerability, or ‘neuroendophenotypes,’ of addiction? “Our current research strongly indicates that there are, and this resonates with findings from human imaging studies.”
A New Wave of Treatments
Everitt says, “despite the great increase in our understanding of brain mechanisms of addiction, new treatments have been slow to emerge, and this is frustrating.” Diminishing the ability of drug cues to elicit craving and relapse is perhaps one way to help people who have stopped using drugs remain abstinent. Some drugs do this in experimental models, but it requires big pharma to engage with this challenge for treatments to be developed — and this hasn’t yet happened.
Another promising approach is to weaken drug memories elicited by drug-associated stimuli. “This may sound odd,” Everitt says, “but it is well established that briefly reactivating a drug memory by presenting drug cues in animals or drug-taking videos in humans, makes drug memories unstable in the brain.”
“We showed that amnestic treatments given in a 4-hour window after retrieval in rats can prevent the restabilization of the memory and so greatly weaken the ability of those cues to elicit drug-seeking subsequently.” This finding has been translated successfully in the treatment of nicotine and heroin addiction but, Everitt notes, “it’s early days and advances will require a much greater understanding of the how to achieve this in people with long and varied histories of drug use.”
An alternative is to increase the activity of the prefrontal cortex that has been decreased by cocaine drug use in animals and humans, with the intention of increasing top-down inhibitory control over maladaptive drug seeking and use. Initial clinical studies have demonstrated that transcranial magnetic stimulation (TMS) of the prefrontal cortex can significantly enhance abstinence in people long addicted to stimulants. “These results are highly promising but require replication in more extensive clinical trials,” says Everitt.
Everitt’s work illuminates some ways that addictive drugs can hijack or alter the brain’s natural programming, leading to potent changes in drug-seeking behavior in vulnerable individuals. Everitt says, “We are unlikely to stop people becoming addicted to drugs, but when they want to stop drug use, animal experimental data have given us very strong indications as to the neural and psychological mechanisms that might be targeted to develop much needed new treatments to help them do so.”
CONTENT PROVIDED BY
BrainFacts/SfN
References
Everitt, B. J., & Robbins, T. W. (2016). Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annual review of psychology, 67, 23–50. https://doi.org/10.1146/annurev-psych-122414-033457
Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews. Neuroscience, 12(11), 652–669. https://doi.org/10.1038/nrn3119
Martinotti, G. (2017, November – _). Transcranial Magnetic Stimulation for Cocaine Addiction (BRAINSWITCH). Identifier NCT03333460. https://clinicaltrials.gov/ct2/show/NCT03333460
Murray, J. E., Belin-Rauscent, A., Simon, M., Giuliano, C., Benoit-Marand, M., Everitt, B. J., & Belin, D. (2015). Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nature communications, 6, 10088. https://doi.org/10.1038/ncomms10088
Torres-Castaño, A., Rivero-Santana, A., Perestelo-Pérez, L., Duarte-Díaz, A., Toledo-Chávarri, A., Ramos-García, V., Álvarez-Pérez, Y., Cudeiro-Mazaira, J., Padrón-González, I., & Serrano-Pérez, P. (2021). Transcranial Magnetic Stimulation for the Treatment of Cocaine Addiction: A Systematic Review. Journal of clinical medicine, 10(23), 5595. https://doi.org/10.3390/jcm10235595
Also In Addiction
Trending
Popular articles on BrainFacts.org