The Past and Promise of Deep Brain Stimulation
- Published28 Jun 2017
- Reviewed28 Jun 2017
- Author Susan Brown
- Source BrainFacts/SfN
In the early 1980s, a few batches of contaminated synthetic heroin triggered severe Parkinson’s disease symptoms among the people who used it. The tragedy would lead to a new therapy that alleviates some of Parkinson’s most disabling symptoms.
A Model of Parkinson’s Disease
In the early 1980s, J. William Langston, a neurologist in California, faced a medical mystery: a patient — who was clearly awake — in a frozen state, unable to move or respond. This 44-year old man appeared to be in the grip of advanced and untreated Parkinson’s disease, which usually strikes people in their sixties. As Langston and his team worked to uncover the cause of what ailed their patient, they found several similar cases around the country. What was the common link between these patients? They had all used a synthetic form of heroin contaminated with a molecule called MPTP.
This single chemical contaminant triggered Parkinson’s disease with such devastating precision that it destroyed the lives of those who had inadvertently used it. However, MPTP’s poisonous character also set in motion a tremendous advance in the understanding and ultimately treatment of Parkinson’s disease.
Fortune Favors the Prepared Mind
More than a decade before Langston discovered those frozen addicts, Mahlon DeLong began recording the activity of single neurons involved in movement. First working in Edward Evart’s lab at the National Institutes of Health, DeLong began his lifelong effort of unraveling the intricacies of a relatively unexplored region deep within the brain called the basal ganglia. At the time, scientists believed it was only critical for movement.
By tracing the pathways of neural signals that stimulate movement and those that inhibit movement as they travel from other parts of the brain through the basal ganglia, DeLong developed a meticulous map of deep neural connections. That model pinpointed a group of neurons in the basal ganglia, called the subthalamic nucleus, where abnormal activity could be the cause of Parkinson’s disease (PD) symptoms.
DeLong had little way to test this hypothesis because he lacked an adequate animal model of PD. That changed overnight when Langston made the link between MPTP and Parkinsonism. Researchers learned that monkeys given MPTP quickly froze and developed tremors. DeLong discovered that monkeys given MPTP recovered when he selectively and very precisely destroyed the subthalamic nucleus.
Destroying Faulty Circuits
Destroying parts of the brain to treat a disease sounds contradictory. But, surgeons did just that through the 1960s. Because some tremor patients who had strokes in the basal ganglia saw their tremors decrease, surgeons attempted to obliterate very focused areas of the basal ganglia. Unfortunately, imprecise surgical techniques, an inability to visualize the brain, and incomplete understanding of the circuits involved, meant surgeons too often caused more harm than benefit.
Throughout the 1960s scientists established that the connected clusters of neurons comprising the basal ganglia degenerated with PD, causing significant depletion of the neurotransmitter dopamine. The surgical approach to treating the disease fell by the wayside by the mid-1970s after physicians demonstrated the dopamine precursor L-dopa could both restore dopamine levels in the brain and alleviate symptoms like tremors and “freezing.”
L-dopa is far from a cure, however. The disease continues to progress, and long-term L-dopa treatment causes serious side effects including involuntary writhing movements. DeLong’s 1987 discovery that MPTP-treated monkeys developed PD symptoms, as a result overactive neurons, proved critical. The neurons DeLong described, which exist in the subthalamic nucleus, function in a circuit that inhibits motion. When these neurons are overactive, the monkeys freeze. Those same monkeys started moving again when DeLong destroyed those neurons.
Around the same time, neurosurgeon and physicist Alim-Louis Benabid, began experimenting with a new kind of brain surgery at Joseph Fourier University in Grenoble, France. Patients for whom medications had failed occasionally opted for surgery as a last ditch effort to suppress their tremors. To guard against undesired effects, surgeons routinely used electricity to stimulate the area they intended to remove as a test of its function.
The standard procedure called for surgeons to send a 50 Hz electrical signal through a thin wire sunk into the brain. While operating on a patient suffering from a disorder called essential tremor, Benabid decided to ramp up the frequency. As the stimulation approached 100 Hz, his patient’s tremor disappeared. When he stopped the current, the tremor returned. The intervention was both effective and reversible.
Blocking Bad Signals
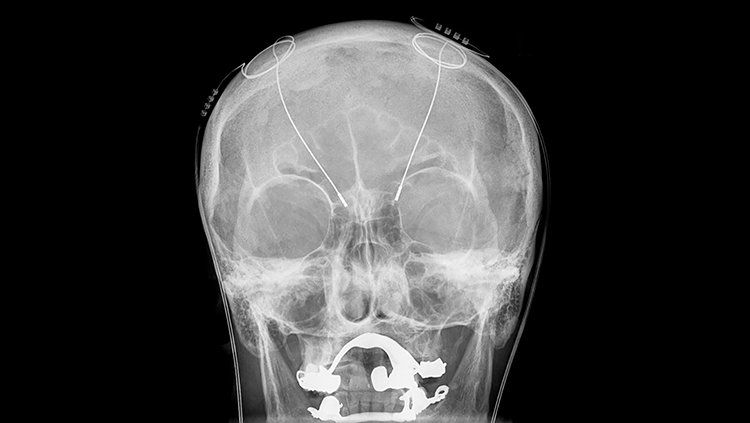
By the time Benabid noticed high-frequency electrical stimulation could control tremor, surgeons had been implanting electrodes into the brains of patients with chronic pain for nearly two decades. Benabid started implanting those devices into the brains of patients with essential tremor or Parkinson’s disease.
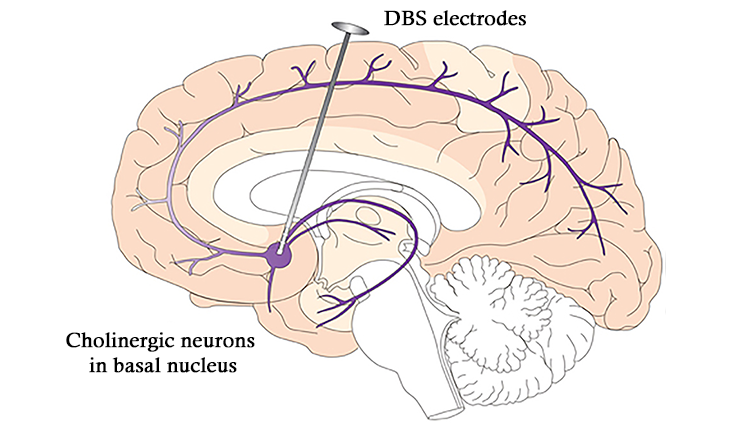
In his first efforts to use deep brain stimulation (DBS) to treat tremor, Benabid targeted the thalamus, a walnut-sized region of the brain that relays both sensory and motor information, while DeLong’s work clearly pointed to the subthalamic nucleus. But, targeting that region was worrisome. Previous surgeries destroying the subthalamic nucleus left patients flailing — a horrific outcome. Benabid reasoned that because DBS could be reversed, if the effects were undesirable, the current could be turned off and the electrode removed.
In the mid-1990s Benabid reported results on three advanced Parkinson’s disease patients who received high-frequency DBS in the subthalamic nucleus. The treatment loosened muscles, eased motion, and allowed the patients to slash their doses of medication. The US Food and Drug Administration (FDA) approved high-frequency DBS in the subthalamic nucleus to treat advanced Parkinson's disease in 2002, and more than 100,000 patients have undergone the procedure since then.
In 2009, FDA approved DBS in a different region of the brain to treat obsessive-compulsive disorder. Physicians are currently exploring how best to use DBS to offer new treatment options for a variety of conditions ranging from Tourette syndrome to treatment-resistant depression and even dementia.
Exactly how deep brain stimulation helps isn’t fully understood, although it seems to recalibrate activity within neural circuitry that has gone awry. That observation has prompted a rethinking of the nature of these disorders. The newest DBS devices can also record neural activity and offer the opportunity to characterize abnormal firing patterns in more precise detail. With continued research support from public and private institutions, scientists may close the loop with devices that both detect and counteract problematic patterns of neural activity for a wide variety of brain diseases.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Hornykiewicz O. A brief history of levodopa. Journal of Neurology. 257(Suppl 2): S249-252 (2010).
Langston W. The MPTP story. Journal of Parkinson’s Disease. 7:S11-S22 (2017).
Shen H. Neuroscience: tuning the brain. Nature. 507: 290-292 (2014).
Strauss E. Deep brain stimulation for Parkinson’s disease. Albert and Mary Lasker Foundation (2014).
Zoghbi H. From anatomy to electrophysiology: clinical lasker goes deep. Cell. 158: 1225-1229 (2014).
Also In Therapies
Trending
Popular articles on BrainFacts.org