Today the world learned that neuroscientist Roger Y. Tsien passed away on August 24, 2016. His life’s work transformed cellular neuroscience.
When you are young and trying to imagine what it must be like to be a renowned scientist, whether you know it or not, you are imagining the life of Roger Tsien. The image that forms in your mind is of someone who is extraordinarily brilliant, and who through singular creativity and ability invents new techniques and discovers secrets about the natural world that leap far into the future to illuminate what was before complete darkness. Roger imagined the possibility of being able to see the invisible--molecules; and to see the molecules going about their work inside living cells.
He imagined being able to watch molecules moving through cytoplasm, squeezing through channels to cross cell membranes, engaging in chemical reactions as they relay signals from the cell membrane to DNA at the heart of the cell’s nucleus. Achieving this fantasy would give scientists the ability to observe molecules at work as they conduct the dazzling array of activities that cells can perform, from transforming into different states and morphing into different shapes, to moving about and interacting with other cells, to releasing substances into the body, and inside the brain operating to build cellular networks of neuronal connections to form memories. Roger did this by attaching molecules that emit fluorescent light to specific molecules in cells that he targeted and was keen to eavesdrop on as they went about their work.
Like a running light attached to a tiny boat at sea in darkness, these fluorescent running lights that Roger designed enabled scientists to look through a microscope while sitting in the darkness of a laboratory room to let their night vision develop and suddenly see the flashes of light inside live cells--to see with their own eyes molecules inside cells moving about, responding to stimulation, interacting with other molecules, and making cells work. One of the important molecules illuminated by Roger’s work is the calcium ion. Cells live in a virtual sea of calcium, but inside cells, free calcium ions are very scarce. This creates an ideal situation for using the movement of calcium ions into cells as a signal, because of the enormous signal-to-noise benefit of a brilliant tracer against darkness. A tiny number of calcium ions entering a cell create a noticeable disturbance, and Nature utilizes this advantage by devising an enormous variety of protein signaling molecules on the cell’s membrane that act as sentinels for specific signals in the cellular environment and marshal an appropriate response.
When these proteins detect the signal they were designed to sense, they open a microscopic channel in the cell membrane to admit a spurt of calcium ions into the cell or they send signals to microscopic cellular storehouses inside the cell that release a burst of calcium ions into the cytoplasm to alert the cell to a specific change in the cell’s environment. This calcium ion signaling is how cells release hormones and neurotransmitters, for example, in response to the appropriate signals. Calcium signaling is the fundamental way neurons communicate with one another using synapses, but cellular signaling by calcium ions is so pervasive, calcium is used for controlling nearly every function a cell can perform, from migrating through tissue to switching on and off specific genes in its nucleus. Roger achieved this ideal by designing molecules that emit light at specific colors when they bind calcium ions, thus glowing like a child’s glow stick to announce where calcium ions had streamed into the cellular cytoplasm and precisely how many ions had done so.
This ability transformed cellular neuroscience and it expanded neuroscience into new realms. In the 1980’s and early 1990s calcium imaging using the tracers Roger invented was very much a black art. The techniques were tricky. The molecules were finicky. It was difficult to get the calcium indicator molecules inside cells and keep them there without damaging the cells and perturbing or destroying the cell’s normal behavior. The imaging technique pushed the limits of the new technology of video microscopy that used night-vision scopes attached to microscopes and coupled to primitive personal computers of the day to detect, amplify, and display fluorescent signals inside cells on a computer screen. Watching their computer displays scientists were confronted with a thicket of cellular artifacts that had to be discerned from true signals as they saw things inside cells that no one had ever seen before.
I will always remember the first time I succeeded in using Roger’s calcium imaging technique to see a neuron firing action potentials. I had traveled to Fort Collins, Colorado to learn this new art from Peter Guthrie in the laboratory of Stan Kater. Our shouts of joy echoed through the halls in the dark lab late at night when Peter and I flipped a switch sending a jolt of electricity to a mouse neuron in cell culture and saw the cell light up like skyrocket. We were seeing a neuron fire electrical impulses with our own eyes by watching the calcium ion tracers that stream through channels in the neuron’s cell membrane when the voltage spikes with each neural impulse. Prior to that we and others could only detect neurons firing by impaling neurons with microelectrodes and watching the blips of voltage streaking across an oscilloscope screen like watching a heart monitor in a hospital.
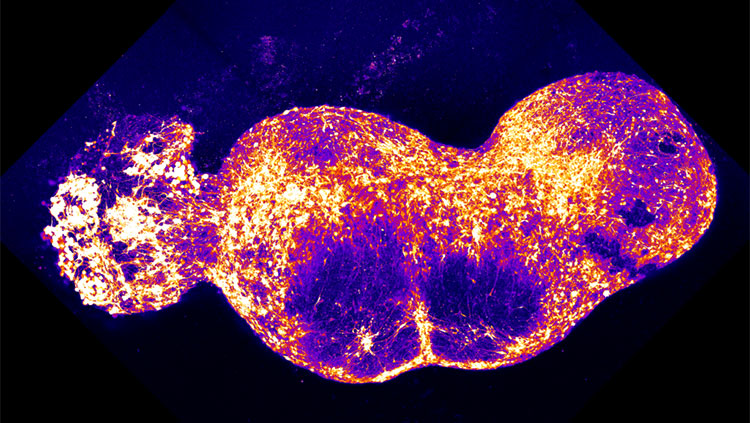
 Fluorescent calcium imaging of DRG neurons and glia responding to action potentials
Fluorescent calcium imaging of DRG neurons and glia responding to action potentials
Later in my own lab I saw something that others were also suddenly startled to find—that when we stimulated neurons to fire impulses, non-neuronal cells, called glia, also responded. Roger’s method had opened neuroscientist’s eyes to “the other brain,” the long neglected function of glia. Glia far outnumber neurons in the brain, but they had been largely ignored because they do not communicate by using electricity. Neuroscientists probing the brain with their microelectrodes were deaf to glial communication--until Roger’s method illumined glia communicating by using waves of calcium ions that relay messages from cell-to-cell and sweep through the brain to interact with and even control communication between neurons at synapses.
Roger then applied his intricate knowledge of organic chemistry and fluorescent molecules with the emerging power of molecular biology to genetically engineer fluorescent tags into specific proteins. By inserting the genes for fluorescent molecules that Nature had provided jelly fish to glow in the deep dark sea, into any gene of interest in a mouse or zebrafish, scientists can now identify and track almost any protein they desire.
 A Golgi stained neuron
A Golgi stained neuron
Roger received the Nobel Prize in 2008. For some achievements, prizes seem inadequate or at least superfluous. Roger’s work permeates every laboratory using molecular biology and advanced imaging to understand cells. This work has advanced major new areas of research and even opened new fields of science, such as optogenetics and neuron-glia interactions. It has led to fundamental understanding about how cells work and how they become diseased. It has provided new cures for disease. Thus Roger’s work swept through scientific laboratories around the world, into hospitals, and even into your local pet store where you can marvel at fluorescent glow fish. I believe that Roger’s technique did for neuroscience at the turn of the 21st century what the Golgi method of staining cells did at the turn of the 20th century.
The Golgi method was a cellular staining technique that enabled scientists to see individual neurons, and thus to begin to understand their structure, diversity, and how they formed circuits and operated. Fluorescent calcium imaging and green fluorescent protein enabled neuroscientists to see all the various types of cells in the body alive and at work. My reference to Dr. Tsien as “Roger” is not disrespectful; it was common practice among neuroscientists. Roger’s brother, Richard W. Tsien, now at NYU, is also a renowned neuroscientist. The brothers each have a record of stellar neuroscience research conducted independently and productively. They have introduced hundreds of new papers into the scientific literature, and they oftentimes collaborated. The fickle probability of chance was at work here, or more likely, the forceful power of family.
Photo credit: Roger Y. Tsien after receiving his Nobel Prize at the Stockholm Concert Hall, 10 December 2008. Copyright © The Nobel Foundation 2008 Photo: Hans Mehlin
Also In Tools & Techniques
Trending
Popular articles on BrainFacts.org