Mapping Your Every Move
- Published26 Mar 2014
- Reviewed26 Mar 2014
- Authors Edvard Moser, PhD, May-Britt Moser, PhD
- Source The Dana Foundation
Edvard Moser is the director of the Kavli Institute and May-Britt Moser is co-director and a founder of the Kavli Institute. In October 2014, they were awarded the Nobel Prize in Physiology or Medicine with John O'Keefe, the director of the Sainsbury Wellcome Centre in Neural Circuits and Behavior at the University College London. This article, authored by Edvard and May-Britt Moser, was originally published in March 2014.
The most advanced surveillance system you will ever find is built into your own brain and nurtured by evolution. It comes equipped with a coding system that stockpiles and maps your lifetime of events in high definition. Through new research tools and insights, scientists are gradually coming to understand this coding system and its intrinsic mathematical principles.
Researchers have long known that different kinds of neurons play different roles in the brain, but only in the past few decades have scientists had access to the imaging and measurement tools they need to see how different neurons react when the brain is challenged with different tasks. We have focused on understanding how the brain helps us navigate in the environment, both because it is intrinsically interesting and because it turns out that navigation—finding our way—is linked to the way we store memories.
We now know that this coding system works like your own air traffic controller—monitoring every movement you make, knowing every step you ever made, and creating links to every event and experience you have had. Essentially, while your brain is making mental maps to help you navigate, it is also overlaying memories—experiences, smells—onto those maps.
From Map to Memories
This ability of the brain to overlay recollections creates a cognitive map—a multilayered collection of memories—rather than a mere cartographic map. It also means that learning how the brain computes navigation is a step toward understanding how networks are built up in the cerebral cortex, the part of the brain that is responsible for imagination, reasoning, and planning—thought processes that make us human.
Further insight into how the brain builds networks in the cerebral cortex can potentially lead to interventions that spare millions of people from the debilitating effects of brain disorders and diseases. The economic consequences are already significant. One study attributes an estimated 35 percent of all burden of disease in Europe to brain disorders. Another study put the total cost of treatment for brain disorders in 2010 at roughly $1.09 trillion. Partly due to economic forecasts, the Obama administration has recognized the importance of funding basic neuroscience research by establishing the National Institutes of Health’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative.
All of these factors underscore why developing insights into the detailed workings of the brain is pivotal for both preventing and treating disorders of memory, and why focusing on the workings of the mammalian spatial-navigation system is so crucial. While what we do is basic research, our work nevertheless examines the very same system that collapses in the case of dementias such as Alzheimer’s disease. Every new piece of knowledge that researchers gather contributes to understanding the puzzle posed by the brain. And we are only just beginning to see the bigger picture.
Encoding Experience on a Map
For a long time psychologists have studied how animals move in and relate to space as a way to understand the larger rules governing how and why we do what we do. Initially, most scholars thought that behavior was simply a matter of stimuli triggering responses. But in 1948, cognitive physiologist Edward C. Tolman suggested a new way to view behavior. The brains of humans and other animals, Tolman said, have a kind of map of their spatial environment, and they encode experience on top of that map. This idea led to the introduction of the cognitive map.
Tolman’s idea was debated but not fully accepted until 1971, when John O’Keefe and John Dostrovsky discovered place cells. Place cells, which fire when an animal is in a specific place, are located in the hippocampus, a paired structure deep inside the brain underneath the cerebral cortex. In experiments, these cells “fired” whenever a rat was in a certain place in its local environment—an indication that there was suddenly something in the brain that actually looked like a map. This finding also helped demonstrate that humans and other animals could make mental maps rather than simply relying on landmarks.
In 1978, O’Keefe and Lynn Nadel took this one step further, proposing that place cells provide animals with a dynamic, continuously updated representation of space and the animal’s position in space. Tolman, it seemed, was right after all.
Discovering Grid Cells
The discoveries of the 1970s gave scientists more clues about what to look for and where to find it. So they looked—and found. One key discovery was head direction cells, which fire when animals face in a certain direction, regardless of the animal’s position.
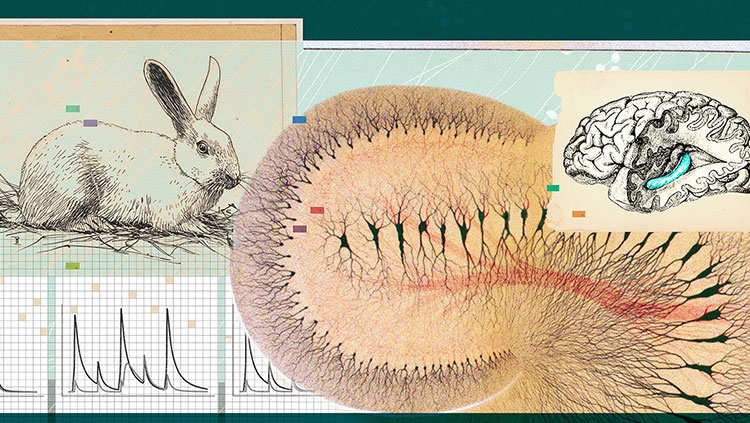
In 2005, our laboratory discovered another kind of cell, called the grid cell, which is located in the entorhinal cortex, right next to the hippocampus. As its name suggests, grid cells create a regular, triangular grid by firing when an animal passes over equally spaced locations. The grid looks very much like the pattern on a Chinese checkers board.
Three years later, our lab and another lab simultaneously reported the existence of yet another type of cell, called a border cell, which fires when an animal is near its environment’s border, such as a wall or an edge.
The collective significance of these findings is that the reactions of the neurons can be matched to what is found in the external world. It is still too difficult to trace other types of complex thinking to their sensory origins. Where information is combined across sensory systems, the firing patterns of the neurons involved are too diffuse for us to detect patterns and relationships to what is happening in the external world.
HM’s Legacy
We know the hippocampus is critical in forming memories, in part because of the unfortunate experiences of an American patient known as HM, who had surgery in 1953 to remove most of his hippocampus as a cure for his extreme epilepsy. The surgery succeeded on one level by reducing his epileptic seizures, but it left him unable to make new memories.
HM is not the only patient whose experiences have illuminated the workings of memory. Many others who suffered injuries to the hippocampus have helped underscore the important connection between this part of the brain and memory formation. We know that one of the first symptoms of Alzheimer’s disease is that patients get lost—and that the first place where a patient’s brain cells begin to die is in the entorhinal cortex.
Essentially, if you have lesions in these areas of the brain, you lose your ability to find your way—and your ability to recall all other types of memories. Memory is deeply and physically connected to our perception and encoding of space. Thus, a detailed understanding of this region of the brain and the operations of its neurons may have the additional benefit of illuminating the mechanisms behind Alzheimer’s disease and related dementias.
New Technologies
As young researchers, what we most wanted to understand was behavior, as well as the origins of complex psychological functions. It’s a question that will take many lifetimes to answer. So by focusing on something more accessible, such as the way space is represented in the brain, we can begin to understand how the brain computes itself, and how external inputs from the senses get into the primary sensory cortex.
Finding these cells required us to use microelectrodes, tiny wires that are thinner than a human hair. They must be correctly placed close to individual neurons in the brains of rats to allow the firing of the neurons to be recorded.
A rat’s brain is the size of a grape. Inside there are about 200 million neurons, each of which has direct contact with approximately 10,000 other neurons. Inside each side of the rat’s grape-size brain are areas that are smaller than a grape seed—collectively, the hippocampus—where memory and the sense of location reside. This is also where we find the place cells—neurons that respond to specific places. But from which cells do these place cells get information?
The answer is to look “upstream” of the hippocampus, to the entorhinal cortex, which feeds information to the hippocampus.
“Listening In” to Neurons
Microelectrodes allow us to listen in on the electrical activity of the cells inside the entorhinal cortex. We have advanced this technique to a level at which we can listen to several hundred cells inside a single rat’s entorhinal cortex. Listening to many hundreds of cells has allowed us to discover that the brain has a number of modules dedicated to self-location. Each module contains its own internal, GPS-like mapping system that keeps track of movement, as well as other characteristics that distinguish it from other modules.
Different modules react differently to changes in the environment. For instance, some scale the brain’s inner map to the animal’s surroundings, while others do not. And the modules operate independently in several ways. The brain can use this independence to create new and varied combinations—a very useful tool for memory formation.
This finding suggests that the ability to make a mental map of the environment arose very early in evolution. All species need to navigate, so that some types of memory may have arisen from brain systems that were initially developed for the brain’s sense of location.
The grid cells in each of the brain’s modules send signals to the place cells in the hippocampus. The combined effect of this grid cell activity creates an activity field in the hippocampus, the place field. This signaling, in a way, is the next step in the progression of signals in the brain. When the environment changes, the different grid modules react differently to the change— firing at new positions in the environment, and the linear summation activates different place cells in the hippocampus.
In practice, this means that the grid cells send a different combinatorial code into the hippocampus in response to the slightest change in the environment. So every tiny change results in a new combination of active cells—cell ensembles that can be used to encode a new memory, and that, with input from the environment, becomes what we call memories.
Neurons Talking
Recent advances in technology have given us opportunities that we could barely dream of only a few years ago. One is the ability to create detailed functional maps that show which neurons talk to each other. We are particularly interested in how grid cells and place cells communicate. The answer to this question will allow us to understand how the deepest parts of the brain are wired together.
When neurons send signals to each other, they share many similarities with electric cables. They send an electric current in one direction—from the “body” of the neuron and down a long arm, called the axon, which extends to the branched arms, or dendrites, of the nerve cell next in line. Brain cells thus get their small electric signals from a whole series of such connections.
A recent technique in our lab involves using a highly modified adeno-associated virus (AAV) as a biological transport system within neurons to better understand which neurons talk to place cells in the hippocampus. The virus is d modified so that it can enter specific neurons and travel upstream through the axon and into the dendrites. We attach a light-sensitive gene to this viral transportation system. This gene integrates itself into the neuron’s DNA and makes the neuron light sensitive. Normally, of course, the neuron is tucked away in the deepest recesses of the brain, in the dark. So this process allows us to install the equivalent of a light switch in a neuronal network.
We used this technique to insert light switches into place cells. Then we inserted optical fibers into a rat’s brain, which enabled us to transmit light to the place cells that had light switches in them. We also implanted thin microelectrodes between the cells to detect the signals sent through the axons every time the light from the optical fiber was turned on. This allowed us to see exactly how the cell-to-cell communication was wired and to map small and large networks within the navigational computation system of the brain.
Mysteries Remain
When we put together all the information, we saw that there is a whole range of differently specialized cells that together provide place cells with their information. The brain’s GPS—its sense of place—is created by signals from place cells to head direction cells, border cells, grid cells, and cells that have no known function in creating location points. Place cells not only receive information about a rat’s surroundings and landmarks, but also continuously update their own movement—an activity that is actually independent of sensory input.
We were surprised to find that cells that have no role in our sense of location actually send signals to place cells, because until now, the specific kinds of brain cells found to be involved in navigation—place cells, head direction cells, and grid cells—all have specific jobs. What is the role of the cells that are not actually part of the sense of direction? They send signals to place cells, but what do they actually do? This remains a mystery.
We also wonder how the cells in the hippocampus are able to sort out the various signals they receive. Do they “listen” to all of the cells equally effectively all the time, or are there some cells that get more time than others to “talk” to place cells?
Speed Cells and Decision-Making
It is easy to forget, as we move effortlessly from home to job, or from job to supermarket to home, the enormous number of processes and steps that make up our ability to navigate. We are now working our way through different aspects of the brain’s navigational system to better understand how all these pieces fit together.
At the moment we are studying what we have dubbed speed cells—cells that react exclusively to the speed of an animal’s movement—and how these types of cells factor in to the navigational equation.
We’re also looking at decision-making. As an animal moves through a labyrinth, it must choose which way to go or what turn to make next. The neurons involved in this decision-making can be found in the prefrontal cortex, which connects to the hippocampus via a small nucleus in the thalamus.
Slowly but surely, we and other researchers are expanding our understanding of other parts of the brain to figure out how everything is connected. And because everything is connected, we are hopeful that as we and others make ever more detailed maps of neural networks, we become more and more likely to find clues that will help prevent and cure brain diseases in the future.
CONTENT PROVIDED BY
The Dana Foundation is a private philanthropic organization that supports brain research through grants and educates the public about the successes and potential of brain research.
Also In Learning & Memory
Trending
Popular articles on BrainFacts.org