Using Breakthroughs in Visual Neuroscience to Treat Diseases
- Published1 Dec 2010
- Reviewed1 Dec 2010
- Source The Dana Foundation
Paul Sieving, M.D., PhD., National Eye Institute, on new ways to look at visual handicaps.
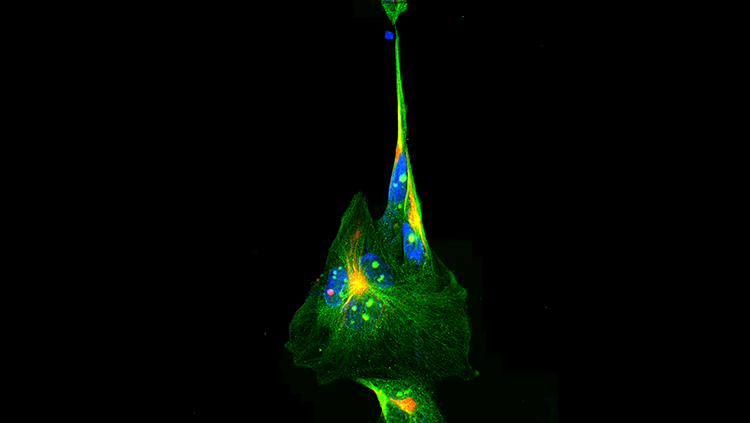
Advances in visual neuroscience during the past 10 years are generating a lot of excitement. The ability to record simultaneously the activity of different clusters of neurons in the eye has greatly improved our understanding of how our neural circuits process and integrate visual signals. For example, recording the impulses from clusters of retinal ganglion cells, which transmit visual input from the eye to the brain, allows researchers to characterize completely the information presented to the visual parts of the brain.
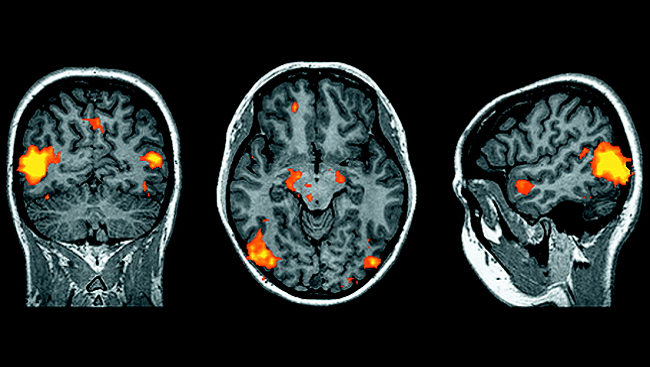
The next research front will involve investigating how neurons interconnect into circuits that control visually guided behavior, such as when we alter our path to avoid an obstacle we see. In the next decade, three recent technical advances will help us learn more about this neural circuitry. First, scientists can now see complex, interconnected brain structures using the Brainbow technique, which employs genetically coded fluorescent proteins that can mark hundreds of neurons with unique colors. Second, two-photon imaging technology can display the dynamic interactions between neurons in real time. Finally, scientists can implant into the brain a grid containing 100 electrodes that deliver signals to a computer, which measures the activity of individual neurons within a larger group.
Scientists can use information about the activity of individual neurons therapeutically. Because the organization of the primate visual system is very close to that of humans, what we learn through ethical studies of nonhuman primates brings us closer to human medical applications. Studies using an array of electrodes implanted in the brain show that monkeys can use their visual system to control an artificial limb remotely, by mental control alone.1 If this ability holds true in humans, it could dramatically improve sensory substitution treatments used for a range of human injuries—for example, better devices for people who have lost limbs due to war or disease. In addition, learning about faulty nerve circuits in the visual system will provide insight into other types of circuit disorders, such as chronic pain and epilepsy.
We have tremendous opportunities to translate what we have learned about visual circuits in the past decade into treatments for neurodegenerative diseases affecting vision, such as retinitis pigmentosa and macular degeneration. These diseases target photoreceptor cells in the retina, which normally process light that becomes an image when electrochemical signals are transmitted through the retina and optic nerve to the brain. The degeneration or death of photoreceptor cells causes loss of vision and blindness, but the other parts of the transmission process—the second-stage retinal neurons—remain intact. Researchers are testing several methods to activate retinal cells by bypassing the nonfunctional photoreceptor cells.
For example, through funding from the National Eye Institute and the U.S. Department of Energy, scientists have developed an artificial retina chip—an electrode array that receives signals interpreted as electrical impulses from a camera. When the array is transplanted into the eye, it stimulates the remaining retinal circuits and transmits the impulses to the brain to enable it to visualize what the camera sees. An alternative strategy involves a microchip with tiny solar cells that convert light energy into electrochemical impulses. And optogenetics, which draws on advances in nanotechnology, uses pulses of light to specifically activate genetically engineered ion channels in retinal cells to initiate the visual pathway.
Researchers are also investigating how to restore vision via cell-based therapies such as stem cell technology and gene therapy. For example, scientists can now induce stem cells to develop into retinal cells, and these cells function correctly when transplanted into an animal with retinal degeneration. The most promising results come from recent clinical trials using gene therapy to treat people with Leber’s congenital amaurosis. People with this condition lack an enzyme required for vitamin A metabolism, and the resultant degeneration of photoreceptor cells causes vision loss. When researchers delivered the missing enzyme to the remaining intact photoreceptors, the patients’ visual sensitivity increased. This was one of the first examples of safe and effective gene therapy.
These advances are beginning to help people with limited vision see better. They are also shedding light on treatments for other neurological disorders. New technologies and scientific breakthroughs afford significant opportunities to expand our knowledge about the visual system and develop applications with therapeutic potential.
CONTENT PROVIDED BY
The Dana Foundation is a private philanthropic organization that supports brain research through grants and educates the public about the successes and potential of brain research.
Also In Archives
Trending
Popular articles on BrainFacts.org