The Value of a Virus
- Published10 Jan 2013
- Reviewed10 Jan 2013
- Author Susan Perry
- Source BrainFacts/SfN
From the nuisance of the common cold to the debilitating symptoms of AIDS, it’s rare to hear a positive story about a virus. But, as it turns out, with the proper manipulations, viruses can do a lot more than cause disease. In fact, scientists use genetically modified viruses to visualize the connections between cells and treat disease.
“Viruses give us an opportunity to manipulate particular cells and regions in the brain — even the whole brain — in unprecedented ways that we couldn’t have done 10 to 15 years ago,” says Beverly Davidson, a neuroscientist at the University of Iowa, who uses modified viruses in her study of inherited neurodegenerative diseases. This technique helps scientists gain important insights into a variety of illnesses, including Parkinson’s disease and some forms of blindness.
Beating viruses at their own game
Viruses — small germs capable of replicating only in other living organisms — have the cunning ability to invade and deliver genetic material to normal cells. When a cold virus, for example, works its way into a cell in the human body, it hijacks the cell’s command center — also known as the nucleus. Once there, the virus instructs the cell to churn out copy after copy of the virus's genetic information. These genetic instructions tell the cell to make more copies of the virus, which go onto infect other cells. Upon recognizing the infection, the body triggers an immune response to fight off the virus.
By swapping out genes from the virus that are harmful to the host cell with other genes, scientists can take advantage of a virus's ability to move from cell to cell and insert new genetic instructions while reducing its ability to cause a dangerous immune reaction.
Once genetically manipulated, the virus simply serves as “a cargo for the genetic material that we place in them,” Davidson says. With the right genetic instructions, scientists coax viruses to carry information capable of illuminating the inner workings of the brain and delivering therapy. Some of the more popular viruses used by neuroscience researchers include rabies, herpes, lentiviruses, and adeno-associated viruses (AAV).
Viruses light up brain pathways
To understand how the human brain works, neuroscientists study how individual cells, or neurons, connect to one another. And therein lies a huge scientific challenge. The human brain has at least 100 billion neurons and their connections number in the hundreds of trillions.
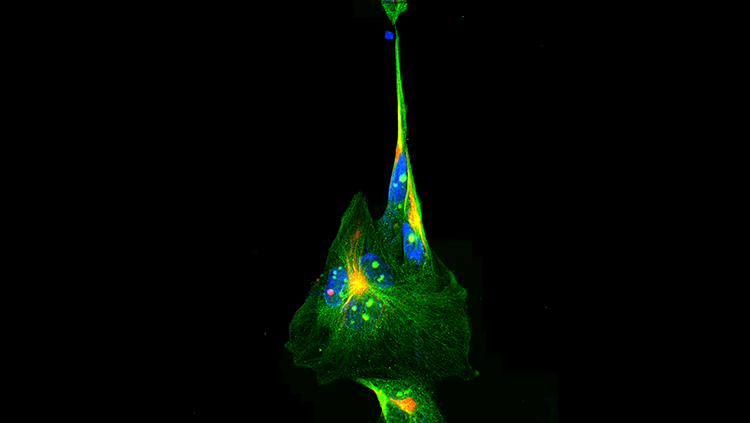
Some viruses, including herpes and rabies, spread specifically between connected neurons. By modifying the genomes of these viruses so that the neurons they infect can be identified and their spread can be controlled, scientists can map the connections between neurons as the virus moves from cell to cell. With these maps, scientists can better understand the pathways of neurons associated with specific brain functions and illnesses.
In one study, scientists used a modified version of the rabies virus to trace the connections between mouse nerve cells that produce dopamine — a brain chemical that is needed for learning actions. The scientists found a direct connection between the dopamine-producing neurons in two regions of the brain, including one area that is a popular target for deep brain stimulation (DBS) — a surgical procedure used to alleviate symptoms of Parkinson’s disease. The findings may help researchers to better understand how DBS works.
Modified viruses help heal
Scientists can also engineer viruses to carry healthy genes to correct abnormal genes affecting the nervous system.
“You can think of the viruses like drugs,” explains Michael Lochrie, director of the Neuroscience Gene Vector and Virus Core facility at Stanford University, which creates genetically modified viruses for researchers to use in their labs. Just like drugs, modified viruses “deliver something to influence biology and benefit the patient.”
One example of the promise of this technique is in treatment of the inherited eye disease Leber’s congenital amaurosis (LCA). LCA is a retinal disease caused by a gene mutation that leads to poor vision at birth and total blindness by adulthood. Scientists engineered AAV to carry a normal version of the gene that is mutated in LCA. When the modified virus was injected into one eye of people with LCA, their vision improved. Recently, a few study participants received the therapy in the second eye, with similar success. Neither the first nor the second treatment triggered an immune reaction.
Future treatments
While gene therapy for neurological conditions remains in its infancy, scientists are hopeful that genetically engineered viruses may one day change the treatment of many neurological disorders and diseases. Neuroscientists continue to explore the use of viruses to deliver gene-based treatments for conditions such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS), brain tumors, pain, and more. In some cases, clinical trials to test the safety and efficacy of the technique are underway.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Bennett J., Ashtari M., Wellman J., et al. AAV2 gene therapy readministration in three adults with congenital blindness. Science Translational Medicine. 4, 120ra15 (2012).
Davidson B.L., Breakefield X.O. Viral vectors for gene delivery to the nervous system. Nature Reviews Neuroscience. 4:353-364 (2003).
Lentz T.B., Gray S.J., Samulski R.J. Viral vectors for gene delivery to the central nervous system. Neurobiology of Disease. Nov; 48(2):179-88 (2012).
Maguire A.M., Simonelli F., Pierce E.A., et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New England Journal of Medicine. 358:2240-2248 (2008).
Watabe-Uchida M., Zhu L., Ogawa S.K., et al. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. Jun 7;74(5):858-73 (2012).
Wall N.R., Wickersham I.R., Cetin A., et al. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proceedings of the National Academy of Sciences. 107(50):21848-21853 (2010).
Also In Archives
Trending
Popular articles on BrainFacts.org