Could the Immune System Be Key to Alzheimer’s Disease?
- Published21 Apr 2021
- Author Esther Landhuis
- Source Knowable Magazine

For nearly 30 years, the hunt for a cure for Alzheimer’s disease has focused on a protein called beta-amyloid. Amyloid, the hypothesis goes, builds up inside the brain to bring about this memory-robbing disorder, which afflicts some 47 million people worldwide.
Billions of dollars have poured into developing therapies aimed at reducing amyloid — thus far, to no avail. Trials of anti-amyloid treatments have repeatedly failed to help patients, sparking a reckoning among the field’s leaders.
All along, some researchers have toiled in the relative shadows, developing potential strategies that target other aspects of cells that go awry in Alzheimer’s: molecular pathways that regulate energy production, or clean up cellular debris, or regulate the flow of calcium, an ion critical to nerve cell function. And increasingly, some of these scientists have focused on what they suspect may be another, more central factor in Alzheimer’s and other dementias: dysfunction of the immune system.
With the field’s thinking narrowed around the amyloid hypothesis, immunological ideas have struggled to win favor — and funding. “There was no traction,” says Malú Tansey, a University of Florida neuroscientist whose work focuses on immunology of the brain. The committees that review grant applications didn’t want to hear about immunological studies, she says.
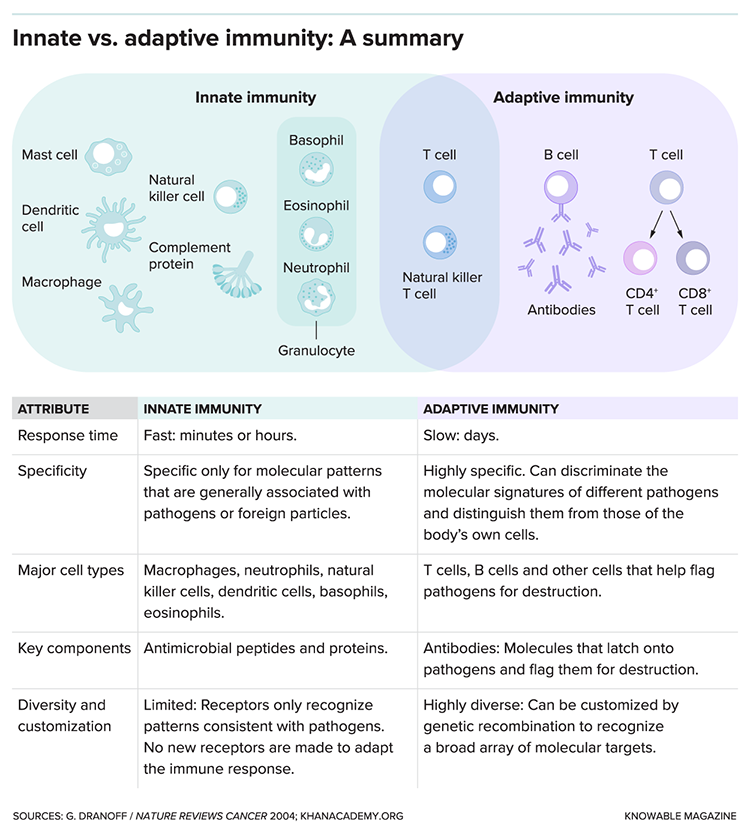
But over the past decade, the immune system connection to Alzheimer’s has become clearer. In several massive studies that analyzed the genomes of tens of thousands of people, many DNA variants that were linked to heightened Alzheimer’s risk turned out to be in genes involved in immunity — specifically, a branch of the body’s defenses known as the innate immune system. This branch attacks viruses, bacteria and other invaders quickly and indiscriminately. It works, in part, by triggering inflammation.
A further connection between inflammation and Alzheimer’s turned up in March 2020, in an analysis of electronic health records from 56 million patients, including about 1.6 million with rheumatoid arthritis, psoriasis and other inflammatory diseases. When researchers searched those records for Alzheimer’s diagnoses, they found that patients taking drugs that block a key molecular trigger of inflammation, called tumor necrosis factor (TNF), have about 50 to 70 percent lower odds of having an Alzheimer’s diagnosis than patients who were prescribed those drugs but did not take them.
This newer wave of studies opened people’s eyes to the idea that the immune system might be a major driver of Alzheimer’s pathology, says Sharon Cohen, a behavioral neurologist who serves as medical director at the Toronto Memory Program in Canada. Over time, Cohen says, researchers began thinking that “maybe inflammation is not just an aftereffect, but actually a pivotal, early effect.”
Tansey is trying to harness this growing realization to develop new therapies. A drug she helped to develop nearly 20 years ago relieved Alzheimer’s-like features in mice and recently showed encouraging results in a small study of people with the disease. “I think we were onto something way back when,” she says.
Early hunch
Tansey got interested in neurodegenerative disease in the late 1990s, while working as a postdoctoral fellow at Washington University in St. Louis. Her research focused on molecules that promote the survival of certain neurons that degenerate in Parkinson’s disease — in lab dish experiments, anyway. But after six years on a meager postdoc salary, and with her husband about to start neurology training at UCLA, she took a job at a biotech company in the Los Angeles area, called Xencor. She tackled a project that the company had on the back burner: designing new drugs to inhibit that inflammatory molecule TNF.
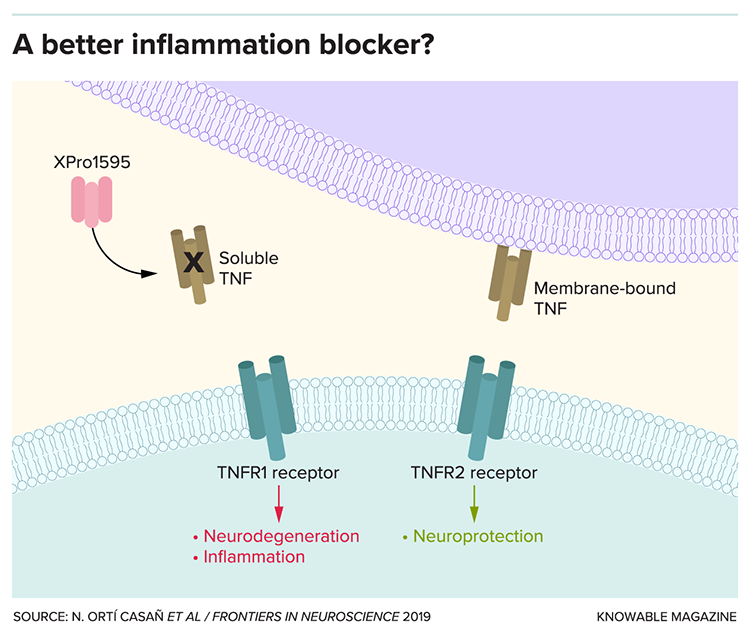
At the time, doctors already used two such drugs to treat autoimmune disorders such as psoriasis and rheumatoid arthritis. But these drugs have harmful side effects, largely owing to TNF’s complicated biology. TNF comes in two forms: one that’s anchored to the membranes of cells, and a soluble form that floats around in the spaces in between. The soluble TNF causes inflammation and can kill cells infected with viruses or bacteria — it’s a necessary job but, in excess, destroys healthy tissues. The membrane-bound form of TNF, on the other hand, confers protection against infection to begin with. The drugs in use at the time inhibited both forms of TNF, leaving people at risk for infections by viruses, bacteria and fungi that typically only cause problems for people with weakened immune systems.
Using genetic engineering, Tansey and her Xencor colleagues designed a drug that prevents this potentially dangerous side effect by targeting only the harmful, soluble form of TNF. It gloms onto the harmful TNF and takes it out of circulation. In tests, injections of the drug reduced joint swelling in rats with a condition akin to arthritis.
This newer wave of studies opened people’s eyes to the idea that the immune system might be a major driver of Alzheimer’s pathology
By the time the work was published in Science in 2003, Tansey had returned to academia, starting up her own lab at the University of Texas Southwestern Medical Center in Dallas. And as she scoured the scientific literature on TNF, she began to think again about those experiments she’d done as a postdoc, on neurons destroyed during Parkinson’s disease. She read studies showing that the brains of Parkinson’s patients have high levels of TNF — and she wondered if TNF could be killing the neurons. There was a clear way to find out: Put the TNF-blocking drug she’d helped to develop at Xencor into the brains of rats that were manipulated to develop Parkinson’s-like symptoms and watch to see what happened.
Her hunch proved correct — the drug slowed the loss of neurons in Parkinson’s rats. And that led Tansey to wonder: Could TNF also be involved in the loss of neurons in other forms of neurodegeneration, including Alzheimer’s disease? Mulling over the nuanced roles of innate immune cells, which seem to help or hurt depending on the context, she started rethinking the prevailing amyloid hypothesis. Perhaps, she thought, amyloid ends up clumping in the Alzheimer’s brain because immune cells that would normally gobble it up get sluggish as people age: In other words, the amyloid accumulated as a consequence of the disease, not a cause.
The double-edged nature of immune activity also meant that our immune systems might, if unchecked, exacerbate problems. In that case, blocking aspects of immune function — specifically, inflammation — might prove helpful.
The idea that blocking inflammation could preserve cognition and other aspects of brain function has now found support in dozens of studies, including several by Tansey’s lab. Using an approach that induced Alzheimer’s-like neurological symptoms in mice, neuroscientist Michael Heneka, a researcher at Germany’s University of Bonn, and his colleagues found that mice engineered to lack a key molecule of the innate immune system didn’t form the hallmark amyloid clumps found in Alzheimer’s.
Tansey and colleagues, for their part, showed that relieving inflammation with the drug Tansey helped develop at Xencor, called XPro1595, could reduce amyloid buildup and strengthen nerve cell connections in mice with Alzheimer’s-like memory problems and pathology. Her team has also found that mice on a high-fat, high-sugar diet — which causes insulin resistance and drives up Alzheimer’s risk — have reduced inflammation and improved behavior on tests of sociability and anxiety when treated with XPro1595.
All told, hints from human genetic and epidemiologic data, combined with growing evidence from mouse models, “was shifting or pointing toward the role of the immune system,” says Heneka, who coauthored a 2018 article in the Annual Review of Medicine about innate immunity and neurodegeneration. And the evidence is growing: In 2019, a study of more than 12,000 older adults found that people with chronic inflammation suffered greater mental losses over a period of 20 years — a clue, again, that inflammation could be an early driver of cognitive decline.
The accumulating data convinced Tansey that it was time to test this idea in people — that “instead of targeting amyloid, we need to start targeting the immune system,” she says. “And it needs to be early.” Once too much damage is done, it may be impossible to reverse.
Targeting innate immunity
Immune-based strategies against Alzheimer’s are already being pursued, but most are quite different than what Tansey was proposing. Companies mostly work with the “adaptive” immune system, which attacks pathogens or molecules very specifically, recognizing them and marking them for destruction. Experimental therapies include antibodies that recognize amyloid and target it for removal.
INmune Bio, in La Jolla, California, is one of several biotech companies taking a different approach: trying to fight degenerative brain disease by targeting the less specific innate immune system. “The immune system is a 50-50 partnership,” says RJ Tesi, the CEO. “If you’re about to have a prize fight, you’re not going to jump in with one hand tied behind your back. Likewise, with Alzheimer’s or cancer, you don’t want to go into the ring with half the immune system being ignored.” To pursue this strategy, INmune Bio bought commercial rights to XPro1595. (Tansey is a paid consultant for INmune Bio but is not involved in any of the company’s trials.)
INmune Bio initially focused on cancer, so when it designed its Alzheimer’s trial, it used a strategy commonly used in cancer drug trials. In Tesi’s view, a key reason that experimental cancer drugs succeed far more often than experimental neurology drugs is the use of molecular disease indicators called biomarkers. These are measures such as genetic variants or blood proteins that help to distinguish patients who, from the outside, may all seem to have the exact same disease, but may actually differ from one another.
By using biomarkers to select participants, cancer researchers can enroll the patients most likely to respond to a given drug — but many neurology trials enroll patients based solely on their diagnosis. And that’s problematic, says Tesi, because scientists are coming to realize that a diagnosis of Alzheimer’s, for instance, might actually encompass various subtypes of disease — each with its own underlying biology and each, perhaps, requiring a different treatment.
In an ongoing trial of XPro1595, INmune Bio aims to enroll 18 people with mild to moderate Alzheimer’s disease, all of whom have elevated levels of biomarkers for excessive inflammation, including one called C-reactive protein. In July, the company reported early data from six participants who were treated with the TNF inhibitor once a week for 12 weeks and assessed for brain inflammation using a specialized magnetic resonance imaging (MRI) technique.
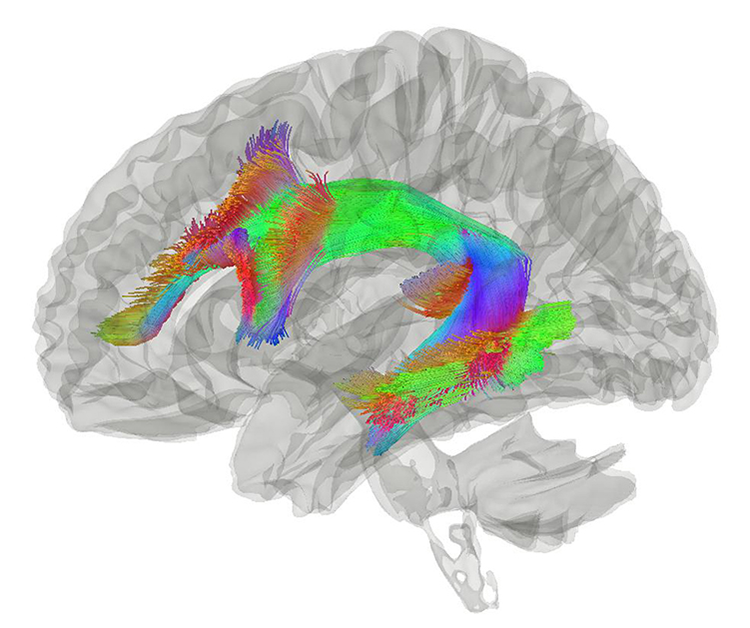
Over the 12-week period, brain inflammation fell 2.3 percent in three participants who received the high-dose TNF inhibitor — compared with a 5.1 percent increase in 25 Alzheimer’s patients whose data were collected previously as part of a major long-term study of Alzheimer’s disease. Three participants who got a low dose of XPro1595 had a smaller — 1.7 percent — increase in brain inflammation. In this small trial, the researchers did not track changes in cognition. But their MRI analysis showed that inflammation was reduced by about 40 percent in a particular bundle of nerve fibers called the arcuate fasciculus that is important for language processing and short-term memory.
“It’s early days,” Cohen says — interim results in just six people. “However, in a small sample size like that, you might not expect to see anything.” Past studies of anti-inflammatory drugs did not show a benefit in Alzheimer’s patients, but scientists are now reexamining these trial failures, Cohen says. “Maybe the idea of the immune system is important, but our therapies were too blunt,” she says.
It’s not just INmune Bio that has researchers excited about the prospect of tinkering with innate immunity to tackle brain disease. Alector, a South San Francisco biotech company, is developing potential therapeutics to activate the innate immune system to fight Alzheimer’s. Some of their experimental drugs are intended to boost the activity of innate immune cells in the brain called microglia. Tiaki Therapeutics in Cambridge, Massachusetts, meanwhile, is using computational methods to identify potential treatments for people with neuroinflammatory diseases who have specific gene signatures. And another company, Shanghai-based Green Valley, is investigating a drug that includes a mix of seaweed sugars that, the company claims, alters gut bacteria to tamp down brain inflammation.
It’s encouraging to see so many different approaches to harnessing the innate immune system to fight Alzheimer’s, Heneka says. He predicts, however, that a variety of treatments will be needed to tackle such a multifaceted, complicated disease.
But Tansey suspects that chronic inflammation is a crucial factor that takes a toll on the brain over the course of many years. Although lowering inflammation will not solve everything, she says, “I think it will buy you a lot. Because it’s the dark passenger of the journey.”
CONTENT PROVIDED BY
Knowable Magazine is an independent journalistic endeavor from Annual Reviews.
Also In Alzheimer's & Dementia
Trending
Popular articles on BrainFacts.org