Insights to the Visual System
- Published7 Jan 2016
- Reviewed7 Jan 2016
- Author Alexis Wnuk
- Source BrainFacts/SfN
New discoveries regarding connections in the retina may provide clues in the search for a treatment for eye disease.
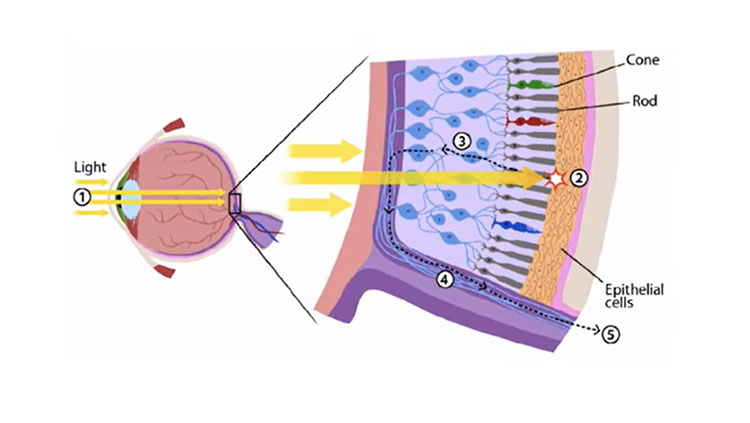
Retinal ganglion cells receive signals from the retina’s light-sensing cells and send the information to the brain for processing. These cells are destroyed in glaucoma, leading to progressive vision loss.
Each of our eyes has a slightly different view of the world around us. Putting these two pictures together depends on the complex wiring that connects the eyes to the brain.
This wiring includes cells called retinal ganglion cells, which receive information from the eyes’ light-sensing cells (photoreceptors) and extend fibers (axons) into the brain. These nerve fibers, which form the optic nerves, transmit information from each eye to the same and opposite sides of the brain, enabling binocular vision.
The axons find their way to the brain during embryonic development. Understanding the factors that guide growing axons — or block their paths — could help scientists regrow nerves injured by stroke or trauma or those lost in blinding diseases like glaucoma.
Visualizing Development
SfN Past President Carol Mason has devoted her research career to learning how neural circuits form in the brain, and her work has revealed how the growing axons of retinal ganglion cells find their way to the correct brain targets.
The journey the axons make is not a straight shot, and they encounter many obstacles along the way. One of these occurs at the optic chiasm, where the optic nerves from each eye meet. At this juncture, the axons from each eye diverge, with some fibers crossing over to the other side of the brain and the rest continuing to targets in the same hemisphere.
In collaboration with Pierre Godement in the 1990s, Mason studied retinal axon growth cones, the tips of growing axons whose finger-like extensions search for the right synaptic targets. After staining retinal ganglion cells in the embryonic mouse brain, Godement filmed the axons as they grew out from the eyes. While all of the retinal axons grew toward the optic chiasm midline, those that did not cross over made a sharp turn and rerouted to the same side of the brain. Moreover, all of the growth cones repeatedly inched forward and retracted before finally crossing through or turning back, suggesting that something in the optic chiasm barred their entry.
Mason’s later research illuminated the factors that direct these behaviors. She found that proteins in the optic chiasm bind to receptors on the axonal tips, triggering a cascade of events that either permits growth or prevents the axons from going forward. While all of the growing axons face this molecular barricade, the fibers that cross over are somehow able to overcome it.
“In order for you to see binocularly … you have to have the proper proportion of cells that go to the same and opposite sides of the brain,” Mason said. In humans, this proportion is roughly half and half.
“We need to understand how these axons develop normally if we’re going to recapitulate growth after injury,” Mason said.
Catalyzing the Search for Cures
Glaucoma, which occurs when the axons of retinal ganglion cells are damaged, is the second leading cause of blindness worldwide, according to the World Health Organization. While it is possible to slow the progression of the disease, there are currently no cures. The retinal ganglion cells ultimately die if their axons are damaged, and the resulting vision loss is permanent.
However, in 2013 the National Eye Institute (NEI) launched the Audacious Goals Initiative (AGI) with the aim of catalyzing research that could lead to cures for glaucoma and other blinding diseases. Over the next 10 to 15 years, the AGI aims to restore vision in these eye diseases by regenerating the retina’s photoreceptors and retinal ganglion cells. The AGI is also focusing on identifying ways of reconnecting axons of injured retinal ganglion cells.
One strategy to prompt proper axon growth and regeneration after injury involves understanding the timing of visual system development. Through her recent research, Mason has learned that the retinal ganglion cells that do not cross the brain’s midline are born during a smaller window of time and express a distinct set of genes that control their differentiation.
“This is exciting because if we’re going to direct stem cells or even actual retinal ganglion neurons to become one type of cell or another, we need to know the steps and the molecules that direct them,” Mason said.
Other researchers in the field are learning about genes that encourage axon growth in the developing nervous system. These genes are shut down in adults but could be stimulated to make axons regrow, she said.
The results of the Audacious Goals Initiative could offer hope to the millions of people affected by stroke, injury, and diseases like glaucoma. They will also have broad implications for the field of neuroscience: “The visual system has not only been a great system to study in terms of learning how we see and how the circuits are set up, but it’s also produced a lot of insights into how we can get any neuron in the central nervous system to regrow,” Mason said.
CONTENT PROVIDED BY
BrainFacts/SfN
Also In Vision
Trending
Popular articles on BrainFacts.org