Neurogenesis: An Overview
- Published21 Jul 2016
- Reviewed21 Jul 2016
- Author Alexis Wnuk
- Source BrainFacts/SfN
The brain’s limited capacity to regenerate triggered the belief that neurogenesis — the birth of new brain cells — ceased after embryonic development. In the latter half of the 20th century, scientists found new cells are born in the brain throughout life. Today this knowledge is helping scientists understand learning and memory.
Challenging Dogma
When the skin is cut, it will repair itself. Broken bones heal just as strong. But damage to the brain and spinal cord tends to be permanent. This limited capacity to regenerate led to the long-held belief that the brains of humans and many other animals couldn’t grow new signaling cells, or neurons. Scientists concluded that the birth of new neurons, or neurogenesis, was limited to embryonic development.
Pioneering research by Joseph Altman in 1962 suggested otherwise. Altman injected adult rats with a radioactive molecule that incorporates into new strands of DNA and as such labels new cells with a small amount of radioactivity. When he examined the rats’ brains he found radioactive cells indicating new neurons had been born in the adult brain.
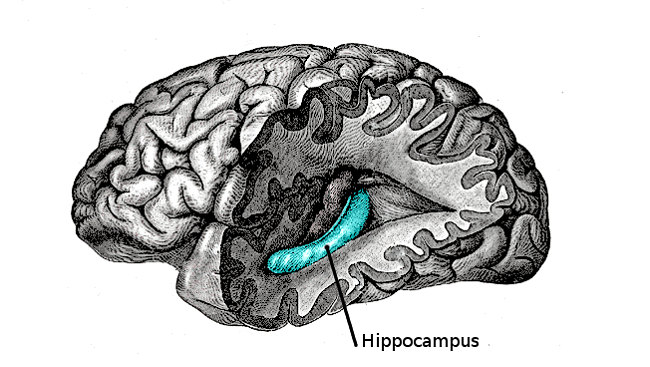
Throughout the 1960s, Altman studied adult rats, finding neurogenesis confined to a handful of areas in the brain — including the hippocampus, a seahorse-shaped structure within each brain hemisphere. Later research would reveal the hippocampus is vital for learning and memory. Specifically, he found newborn cells in a part of the hippocampus called the dentate gyrus. He also discovered neurogenesis in the layer of tissue lining the ventricles, the fluid-filled cavities in the brain.
Even so, the scientific community remained skeptical of the findings because the radioactive labeling didn’t target neurons specifically and the imaging technology of the day couldn’t differentiate neurons from other types of brain cells. In addition, conventional wisdom held that a set number of neurons in the brain wired up together to form circuits. How would it be possible to learn or remember if new neurons were constantly being added to these circuits?
In the 1980s, neurobiologist Fernando Nottebohm provided more conclusive evidence of neurogenesis. Using the same radioactive labeling, he found new cells were born in the brains of songbirds and, going one step further, he found the cells conducted electrical impulses, definitively proving they were neurons.
But despite a growing body of studies like these, it wasn’t until the development of more sophisticated tools in the 1990s that neurogenesis moved to the fore.
New Tools Offer a Clearer Picture of Neurogenesis
In the 1990s, the identification of stem cells in the adult mouse brain demonstrated that unspecialized, precursor cells divided to produce new neurons. New tools for labeling and imaging neurons further propelled the field of neurogenesis.
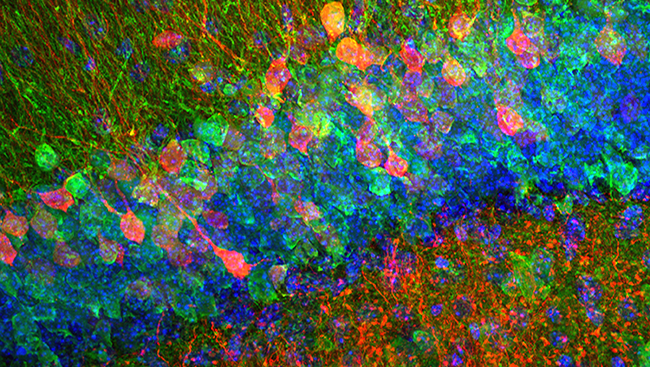
One of these tools employed a non-radioactive molecule called bromodeoxyuridine, or BrdU. Like its radioactive predecessor, BrdU incorporates into new strands of DNA during cell division. Scientists could view new cells harboring BrdU by bathing slices of brain tissue in a solution containing fluorescent markers and using a microscope that captures fluorescence. Improvements in confocal microscopy provided higher-resolution pictures of fluorescently-labeled cells in the brain. With these new tools, scientists proved two things: new cells were born in the brain and some of them were neurons.
In 1996, researchers found that while the rate of neurogenesis in the brain plummeted as rats aged, it was never abolished completely. And in 1998, a landmark study offered the first evidence of neurogenesis in the adult human brain.
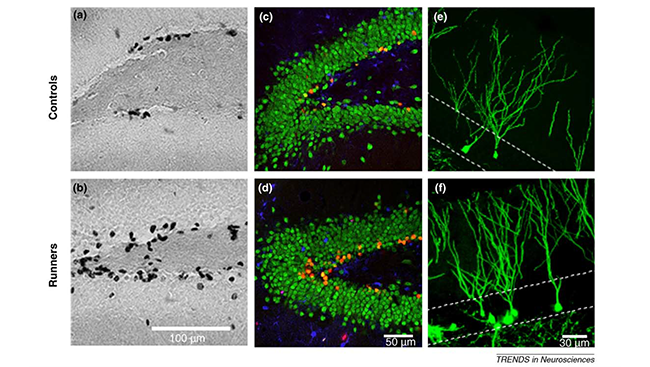
A slew of other studies during this time demonstrated neurogenesis didn’t just vary with age – it could be modified by experience and environment. Stress diminished neurogenesis in tree shrews, while exercise and enriched environments boosted neurogenesis in rats.
In 2002 researchers at The Salk Institute in California visualized and recorded newborn neurons in living mice, something which hadn’t been possible before. The researchers watched as over a period of several months the new cells grew and began to look and behave like mature neurons, forming connections (synapses) and firing electrical impulses.
Neurogenesis was a fact, both in lab animals and people. It declined with age, but could be enhanced by cognitive enrichment and exercise. And it produced functional neurons. But an important question lingered: what role did neurogenesis play in cognition? In the following decade, answers emerged and with them, implications for aging and cognitive health.
Insights for the Aging Brain
Identifying neurogenesis in the adult human brain was a revelation. Still, its mere existence didn’t establish whether adult neurogenesis made a significant contribution to cognition and, if it did, could it be enhanced to benefit the aging brain?
Research offered important insight into neurogenesis, exercise, and learning in 2005 when scientists discovered running on a wheel boosted neurogenesis in older mice as compared to mice that were sedentary. What’s more, running mice performed just as well as younger mice on a spatial learning task.
A discovery in 2009 demonstrated why the brain needs neurogenesis in adulthood. Adult neurogenesis takes place only in two specific areas including a section of the hippocampus called the dentate gyrus – a region key to the ability to discriminate very similar memories like remembering where you parked your car today as opposed to yesterday. Researchers found blocking neurogenesis in mice impaired their ability to remember similar locations in a maze.
A groundbreaking study in 2013 documented the extent of neurogenesis across the human lifespan. Using carbon dating, the researchers estimated the age of hippocampal neurons in postmortem brain samples and constructed a model of hippocampal cell turnover over the lifespan. They determined that there is substantial neurogenesis in the human hippocampus suggesting newly-born neurons make a significant contribution to brain function.
It also suggested enhancing neurogenesis may stave off cognitive decline. Numerous studies indicate the benefits of exercise go beyond cardiovascular health to include preserving cognitive function and mitigating brain atrophy in aging. In addition to increased blood flow and production of growth factors in the brain, neurogenesis may be one way by which exercise exerts its neuroprotective effects.
More than 50 years in the making, the story of neurogenesis began with Joseph Altman’s small study in 1962. With new tools and technologies at their disposal, a new generation of scientists are revealing truths about neurogenesis and the aging brain. With continued support from public and private institutions, scientists will continue to build on these discoveries and identify potential strategies to prevent cognitive decline in aging.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Altman J. Are new neurons formed in the brains of adult mammals? Science. 135: 1127-1128 (1962).
Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of Comparative Neurology. 124(3): 319-335 (1965).
Bakker, A, Kirwan CB, Miler M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 319: 1640-1642 (2008).
Barber SE, Clegg AP, Young JB. Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age and Ageing. 41(1): 5-8 (2012).
Clelland CD, Choi M, Romberg C, Clemenson Jr GD, Fragniere A, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 325(5937): 210-213 (2009).
Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. PNAS. 107(5): 2367-2372 (2010).
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A, Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 4(11): 1313-1317 (1998).
Goldman SA. Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proceedings of the National Academy of Sciences. 80: 2390-2394 (1984).
Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. The Journal of Neuroscience. 17(7): 2492-2498 (1997).
Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 197(4308): 1092-1094. (1977).
Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. The Journal of Neuroscience. 22(3): 635-638 (2002).
Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. The Journal of Neuroscience. 18(9): 3206-3212 (1998).
Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of Neuroscience. 16(16): 2027-2033 (1996).
Lois C, Kelsch W. Adult neurogenesis and its promise as a hope for brain repair. Frontiers in Neuroscience. 8(165): 1-3. (2014).
Ming G, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 70(4): 687-702 (2011).
Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 225: 1046-1048 (1984).
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 153: 1219-1227 (2013).
van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. PNAS. 96(23): 13427-13431 (1999).
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 415: 1030-1034 (2002).
van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of Neuroscience. 25(38): 8680-8685 (2005).
Also In Brain Development
Trending
Popular articles on BrainFacts.org