Vision and Gene Therapy
- Published4 Apr 2009
- Reviewed25 Mar 2014
- Author Debra Speert, PhD
- Source BrainFacts/SfN
The discovery of a vitamin's crucial role in vision helped pave the way for gene therapy advances.
Vision loss and eye disease affect 3.6 million Americans and cost the United States $68 billion each year. However, advances in vision research are helping to combat some types of eye disease. An unexpected finding decades ago — the crucial role of vitamin A in the visual system — and the genetic revolution that began with the decoding of the human genome combined to create one of the earliest success stories for gene therapy and hope for vision restoration in people.
Research identifies the chemical and genetic bases of vision
In the 1930s, a researcher named George Wald discovered the vital role of vitamin A in the visual system, but the connection between diet and vision had been known for centuries. Ancient Egyptians recognized that night blindness could be a symptom of a poor diet. During World War I, researchers linked nutritional night blindness to deficiency of vitamin A, a newly discovered vitamin obtained from food.
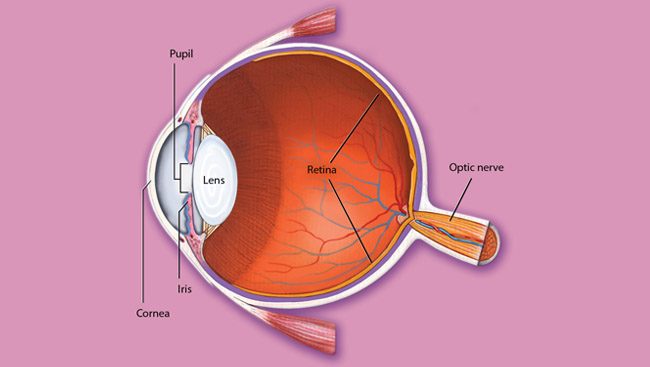
Why does the lack of vitamin A impair vision? Researchers had previously isolated a purple pigment from rod photoreceptors, light-sensitive nerve cells in the retina that are important for night vision. Wald found that the pigment, rhodopsin, consisted of both a protein and a light absorbing chemical related to vitamin A. He further identified a “visual cycle” that is essential for vision. First, light separates the vitamin A molecule from the protein and changes its shape, leading to initiation of neural signals from the eye to higher brain centers. Then, the vitamin A molecule is “recycled” — restored to its original shape and reunited with its protein partner. Research showed that the restoration of vitamin A is necessary for the eye’s continual absorption of light. Wald’s discovery earned him the Nobel Prize.
Genetics of Eye Disease
While research by Wald and others identified the basic mechanism of vision more than 75 years ago, the recent decoding of the human genome has helped determine how vision can be affected by disease. In the past 30 years, researchers have identified more than 400 genes that cause eye diseases. These inherited eye disorders comprise a major portion of all genetic diseases — nearly a fifth of all genes known to be related to any disease cause eye disorders. By shedding light on the genetic cause of disease, this research has opened the door to new treatment possibilities for disorders once believed to be permanent.
Identifying a gene involved in childhood blindness
Leber congenital amaurosis (LCA) is a set of genetic eye diseases that affects infants and children. In fact, LCA is one of the most common causes of congenital vision loss in children. Infants with LCA are born with visual impairments that become more severe through adolescence, leading to complete blindness in adulthood.
To date, researchers have identified 13 gene mutations that can each result in LCA. One form of LCA is caused by a mutation in the gene RPE65 [JC1] , which is linked to vitamin A and the visual cycle. Researchers at the National Eye Institute identified RPE65 in 1993, and shortly thereafter found that its mutation caused a form of LCA. Because it was the second gene found to be associated with LCA, the researchers named the specific disease caused by RPE65 mutation LCA2.
How is RPE65 important for vision? To answer this question, researchers generated mice that lacked the RPE65 gene. They found that these mice had impaired vision because they were unable to recycle vitamin A in the visual cycle. Without the vitamin A molecule, rhodopsin could not be made, and without rhodopsin, rod photoreceptor cells could not respond to light, although they remained intact. Therefore, the research suggested that people with LCA2 may have all the machinery for vision — they just need a healthy copy of the RPE65 gene to restart the visual cycle.
Hijacking a Virus
Researchers have developed ways to deliver healthy versions of affected genes to correct some genetic disorders. For RPE65 , they used a virus to get the gene into photoreceptors. Viruses, like the flu or the common cold, take over a cell’s machinery to reproduce their own genetic material and make more viruses. Researchers chose a virus that does not make people sick, removed its infective parts, and used it as a carrier for the healthy gene. After inserting the human RPE65 gene, they prepared to test the modified virus — an RPE65 gene therapy agent.
Research Helps Blind Dogs See
Before testing the gene therapy agent in humans, researchers needed to check its safety and effectiveness in animals. They found an appropriate animal model of LCA2 in Briard dogs, a breed that is prone to a condition that resembles LCA. Researchers injected the eyes of sick dogs with the modified virus that carried the RPE65 gene. Once treated, dogs that had been visually impaired their whole lives behaved as if they could see more clearly, and their eyes responded to light. Dogs that had once bumped into objects in their paths were able to navigate through obstacle courses within weeks of treatment.
Translating Animal Research to Humans
In 2007, just 15 years after RPE65 was cloned and 70 years after rhodopsin was discovered, three groups of researchers tested this gene therapy agent in humans with LCA2. Unlike most medications, which are secreted from the body and need to be taken regularly, most gene therapies last for extended periods of time, suggesting that just one treatment with RPE65 gene therapy might result in long-lasting improvement.
In clinical trials performed in the United States and United Kingdom, independent research teams found that RPE65 gene therapy could improve sight in affected children and young adults, to the extent that they could see to find their way around more safely and quickly.
These studies indicate a new direction in treating genetic eye diseases, including other forms of LCA. But their implications extend beyond the eye. Every day, research in labs across the world helps uncover basic biological and chemical processes, like George Wald’s discovery of the visual cycle. With this knowledge as an important foundation, as researchers identify gene mutations that cause disease, they are increasingly able to treat them, potentially allowing clinicians to transition quickly from patient diagnosis to treatment.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Therapy. 15(11): 849-857 (2008).
Bainbridge JWB, Smith AJ, Barker SS, Robbie S, Henderson R, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. New England Journal of Medicine. 358: 2231-2239 (2008).
Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Human Molecular Genetics. 11(10):1169-76 (2002).
Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, et al. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. Journal of Biological Chemistry. 268(21): 15751-1575 (1993).
Narfström K, Katz ML, Ford M, Redmond TM, Rakoczy E, et al. In vivo gene therapy in young and adult RPE65-/- dogs produces long-term visual improvement. Journal of Heredity. 94(1):31–37 (2003).
Stieger K, Lorenz B. Gene therapy for vision loss -- recent developments. Discovery Medicine. 10(54):425-33 (2010).
Weleber RG. Inherited and orphan retinal diseases: phenotypes, genotypes, and probable treatment groups. Retina. 25(8 Suppl):S4-S7 (2005).
Also In Archives
Trending
Popular articles on BrainFacts.org